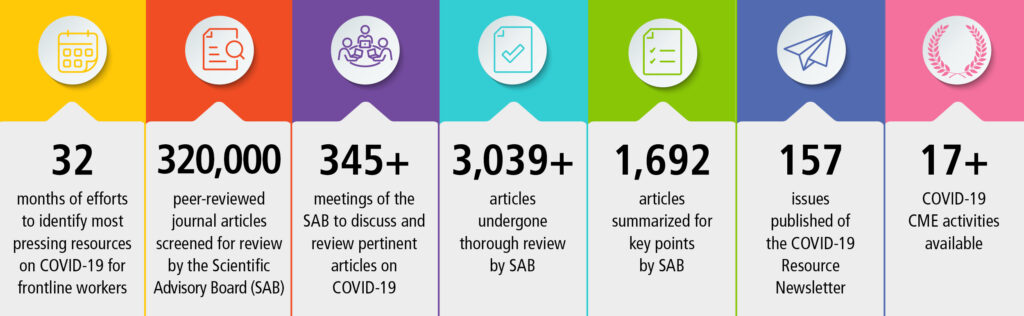
Following declarations from the US Department of Health and Human Services and World Health Organization ending the COVID-19 Public Health Emergency, the IARS COVID-19 Scientific Advisory Board (SAB) concluded its review of the scientific literature about SARS-CoV-2 in August. The SAB has reviewed more than 3,100 journal articles and published 1,076 article reviews over the past 42 months. It has been an enormous commitment from the SAB, and the IARS owes our dedicated physician volunteers a huge debt of gratitude for their unwavering participation in this initiative.
The IARS COVID-19 Scientific Advisory Board (SAB) has screened newly published peer-reviewed articles from respected journals to identify those of greatest clinical and scientific relevance to anesthesiologists, intensivists, related specialists and investigators. Our open-access newsletter provides a link to each highlighted article along with a short summary of key points. The SAB does not include any information from news media, social media, or scientific articles lacking full peer-review such as pre-prints.
To search by keyword, select Ctrl + F on a PC and Command + F on a Mac. Then, enter keyword and Enter.
The material on this website is provided for informational purposes and does not constitute medical advice. New knowledge is added daily and may change over time. Opinions expressed should not be construed as representing IARS policy or recommendations. References and links to third parties do not constitute an endorsement or warranty by IARS.
Newsletter: Issue 163, May 22, 2023
Following the recent declarations from the US Department of Health and Human Services and World Health Organization ending the COVID-19 Public Health Emergency, the IARS COVID-19 Scientific Advisory Board (SAB) is pausing its review of the scientific literature about SARS-CoV-2. Although COVID-19 remains a concern for frontline healthcare providers, it has taken a back seat to more pressing concerns. Moreover, the publication of new COVID-19 information relevant to frontline providers has waned significantly. The SAB will reconvene in three months to reassess the future of this endeavor more permanently. In the meantime, today’s issue of the COVID-19 newsletter will be the last one you will see for at least awhile. The articles and summaries compiled on our COVID-19 resource website will continue to be available should you need access to that content.
With the publication of the two article summaries in this issue of the newsletter, the SAB has reviewed more than 3,100 journal articles and published 1,076 article reviews over the past 39 months. It has been an enormous commitment from the SAB, and the IARS owes our dedicated physician volunteers a huge debt of gratitude for their unwavering participation in this initiative. It’s time for us to allow them to return to their well-earned retirement lives unencumbered by the responsibility of reviewing articles, drafting summaries, and meeting regularly to discuss and debate the relevance to frontline care providers.
We hope you have found the COVID-19 newsletter to be an informative, reliable, and valuable resource during this very difficult period for clinicians, patients and healthcare. Although in healthcare there is no such thing as “business as usual,” we look forward to being able to focus on the challenges of clinical care and research discovery without the additional burdens of COVID-19.
As always, we welcome your feedback on this newsletter and initiative. Please email [email protected] with any comments.
- Prevalence and Characteristics Associated With Post-COVID-19 Condition Among Nonhospitalized Adolescents and Young Adults. 3/30/23. Selvakumar J. JAMA Netw Open.
This study calls into question the usefulness of the WHO definition of post-COVID condition (PCC). The goal of this prospective Norwegian study conducted during the Alpha-dominant wave was to define the prevalence and risk of post-COVID condition (PCC) in young outpatients, a little-studied population. “This cohort study included 382 SARS-CoV-2–positive individuals and a control group of 85 SARS-CoV-2–negative individuals aged 12 to 25 years who were assessed at the early convalescent stage and at 6-month follow-up.” At inclusion and follow-up participants underwent a clinical interview, complete physical exam, measurement of vital signs, spirometry, electrocardiogram (ECG) including heart rate variability, cognitive function tests, blood tests including viral antibodies, cytokines and inflammatory markers, and questionnaires. Results: “When applying the World Health Organization case definition of PCC, prevalence at 6 months was 49%, but was also comparably high (47%) in the control group. PCC was not associated with biological markers specific to viral infection, but with initial symptom severity and psychosocial factors.” The main risk factor for PCC was symptom severity at baseline (RR, 1.41), and correlated with personality traits. Female sex, low physical activity, recent negative life events and loneliness were also factors.
SAB Comment: Our group found this study of interest, despite weaknesses that include a relatively small control group, and a question of whether self-selection may have played a role, given the number of hours required for the two evaluations. Additionally, although neuropsychiatric factors may be implied, we note that thorough neuropsychiatric testing was not part of the study. On the other hand the design was prospective and testing relatively complete compared with many if not most studies of post-COVID conditions. We hope it comes to the attention of the WHO. - Expert consensus statement on venovenous extracorporeal membrane oxygenation ECMO for COVID-19 severe ARDS: an international Delphi study. 5/2/23. Rabie AA. Ann Intensive Care.
The modified Delphi technique was used by 22 international extracorporeal membrane oxygenation (ECMO) experts worldwide to reach consensus on 14 general statements based on the most recent findings of the evolving published research. These 14 statements were in four domains (clinical, operational, and logistic ECMO management and ethics) and were formulated to guide next-generation ECMO providers during future pandemic situations. Two example statements are, 1) “The duration of invasive mechanical ventilation (IMV) before considering ECMO should not be used as a primary determinant for ECMO candidacy,” and 2) “There is no validated evidence-based scoring system to predict the outcome for COVID-19 patients receiving ECMO. Therefore, available scoring systems previously used for non-COVID-19 patients should not be used for COVID-19 patients as a prognostic tool.” - Below you will find a list of additional articles and resources selected for the IARS COVID-19 Resource website, all of which include a summary with major takeaways and are searchable by topic and date posted on the website:
The material on this website is provided for informational purposes and does not constitute medical advice. New knowledge is added daily and may change over time. Opinions expressed should not be construed as representing IARS policy or recommendations. References and links to third parties do not constitute an endorsement or warranty by IARS.
Previous COVID-19 Newsletters
Newsletter Issue 162, April 25, 2023:
 Effectiveness of nirmatrelvir-ritonavir in preventing hospital admissions and deaths in people with COVID-19: a cohort study in a large US health-care system. 3/18/23. Lewnard JA. Lancet Infect Dis.
Effectiveness of nirmatrelvir-ritonavir in preventing hospital admissions and deaths in people with COVID-19: a cohort study in a large US health-care system. 3/18/23. Lewnard JA. Lancet Infect Dis.
This is an observational outpatient study using electronic record analysis of the Southern California Kaiser Permanente HCS to further determine the effectiveness of nirmatrelvir–ritonavir (Paxlovid) in preventing hospital admissions and death within 30 days of a positive SARS-CoV-2 PCR test. Between April and October 2022, cases were matched by vaccination history, comorbidities, health care seeking trends and BMI in addition to age, sex and clinical status and patients who were tested within 5 days of symptom onset were analyzed separately. A total of 7,274 Paxlovid recipients were matched with 125,152 non-recipients and demonstrated an overall effectiveness in preventing hospital admission of 54% which increased to 80% among a smaller cohort treated within 5 days of symptom onset and to 90% when Paxlovid was dispensed on the day of a positive PCR test result. The authors discuss the etiology of 7 recently published observational studies conducted in the USA, Israel and Hong Kong, where Paxlovid effectiveness ranged from 21-79% in outpatients, and conclude that in a setting with high levels of vaccine uptake, Paxlovid is highly effective, particularly when given early.- Real-world use of nirmatrelvir-ritonavir in outpatients with COVID-19 during the era of omicron variants including BA.4 and BA.5 in Colorado, USA: a retrospective cohort study. 2/13/23. Aggarwal NR. Lancet Infect Dis.
A retrospective cohort study examined the real-world effectiveness of nirmatrelvir–ritonavir among high-risk outpatients with COVID-19 during the Omicron BA.2 and BA.2.12.1 (from March 26 to June 18, 2022) and BA.4 and BA.5 (from June 19 to Aug 25, 2022) waves in Colorado, USA. After propensity-score matching, 7168 patients treated with nirmatrelvir–ritonavir and 9,361 untreated controls were included for analysis. Outpatient use of nirmatrelvir–ritonavir reduced the odds of 28-day all-cause hospitalization from 1.4 to 0.9%, a clinical benefit that was consistently observed during both Omicron BA.2 and BA.2.12.1 and BA.4 and BA.5 predominant periods. All-cause mortality following treatment with nirmatrelvir–ritonavir was 5 times lower in the treatment group (2 deaths vs.15 in the non-treatment group or 0.03% vs. 0.16% in the non-treatment cohort). Fewer emergency department visits following treatment suggests that clinically significant rebound requiring urgent medical care was not observed more frequently among users of oral antivirals.
An accompanying editorial from Hong Kong stresses the ongoing need for real-world studies for two major reasons: 1. Recombinant variants of Omicron – especially XBB.1.5 and BQ.1.1 – continue to emerge posing an imminent threat to public health, due their even greater immune evasion capabilities than BA.5. 2. Such studies remain relevant when assessing cost-effectiveness for different therapeutic strategies and their prioritization among various patient populations, as the number needed to treat to prevent one case of severe COVID-19 might also increase as population immunity grows.
SAB Comment: Using the author’s results, the number of patients necessary to treat to prevent one hospitalization is 200, which is consistent with an absolute risk reduction of 0.5%. - Association of Treatment With Nirmatrelvir and the Risk of Post-COVID-19 Condition. 3/23/23. Xie Y. JAMA Intern Med.
A Veterans Administration cohort study, consisting of 87% men, to examine whether nirmatrelvir in the acute phase of SARS-CoV-2 infection lowers the risk of post-COVID-19 condition (PCC) or long COVID, defined as persistence of symptoms beyond 90 days past the acute episode. The authors identified 35,717 veterans who had been treated in 2022 with nirmatrelvir within 5 days of a positive COVID-19 test and 246,076 controls who had not received any antiviral or antibody treatment and who had at least one risk factor for progression to severe COVID-19. Using inverse probability weighting, the authors determined that nirmatrelvir reduced the relative risk of developing 10 of 13 prespecified symptoms of PCC by 26% (relative risk, 0.74; 95% CI, 0.72-0.77) at 180 days. Nirmatrelvir was also associated with decreased risk of death (47%) and hospitalization (24%). These results were independent of vaccination status or prior infection or re-infection.
SAB Comment: This study strongly supports nirmatrelvir administration during acute COVID-19 for at risk patients to mitigate PCC. - Maternal third dose of BNT162b2 mRNA vaccine and risk of infant COVID-19 hospitalization. 3/24/23. Lipschuetz M. Nat Med.
This is a retrospective analysis of all live-born infants delivered in Israel between 24 August 2021 and 15 March 2022 to estimate the effectiveness of the third maternal Pfizer COVID-19 booster dose versus the second dose against infant COVID-19-related hospitalizations. Among 48,868 live-born infants included in the analysis, rates of COVID-19 hospitalization during the first 4 months of life were 0.7%, 0.6% and 0.4% in the unvaccinated, two-dose and three-dose groups, respectively, supporting clinical and public health guidance for maternal booster vaccination to prevent infant COVID-19 hospitalization. - Symptom and Viral Rebound in Untreated SARS-CoV-2 Infection. 2/21/23. Deo R. Ann Intern Med.
This retrospective analysis of 563 placebo recipients (enrolled in ACTIV-2/A5401 randomized control trials of treatments for mild/moderate COVID-19) sought to characterize symptom and viral rebound in an untreated population. Patients, enrolled between November 2020 and July 2021 (pre-Omicron), were mostly unvaccinated. Patients reported daily scores for 13 symptoms over 28 days, and nasal swab quantitative viral RNA measurements were taken for days 1-14, 21, and 28. Symptom rebound occurred in 26%, was brief, and was associated with female sex, high risk factors for severe COVID, short time since symptom onset, and higher symptom score at study onset. Viral rebound occurred in 31%, was also brief, was not associated with high risk for severe COVID, but was associated with a high viral load at study onset. Both symptom and viral rebound occurred in only 3% of subjects. - COVID-19 bacteremic co-infection is a major risk factor for mortality, ICU admission, and mechanical ventilation. 1/23/23. Patton MJ. Crit Care.
A multicenter (UAB and Ochsner-LSU Shreveport systems), demographically diverse, cohort study of adult community-acquired bacteremic co-infection to date is lacking. This retrospective study evaluated bacteremic co-infection using 48-h post-admission blood cultures. Groupings: (1) confirmed (positive blood cultures), (2) suspected (negative culture with 2 or more doses antimicrobials administered), and (3) no evidence (no blood cultures obtained). The primary outcomes were in-hospital mortality, ICU admission, and mechanical ventilation. Cohorts:13,781 COVID-19 inpatients (2020 to 2022). Results: confirmed (2.5%), suspected (46%), no evidence (51.5%). The comparison cohort: 99,170 pre-COVID inpatients (2010-2019). An easily available elevated neutrophil-to-lymphocyte ratio greater than or equal to 15 (+ or – steroids) within 48-h of admission was a key marker of co-infection. Co-infection posed the greatest risk for in-hospital mortality, ICU admission and mechanical ventilation. Co-infection mortality (24%) with COVID far exceeds pre-pandemic inpatients (5.9%) and is consistent across alpha, delta, and omicron. - Below you will find a list of additional articles and resources selected for the IARS COVID-19 Resource website, all of which include a summary with major takeaways and are searchable by topic and date posted on the website:
- COVID-19 Vaccine Effectiveness Against Omicron Infection and Hospitalization. 3/323. Piché-Renaud PP. Pediatrics.
- Post-acute sequelae after SARS-CoV-2 infection by viral variant and vaccination status: a multicenter cross-sectional study. 3/11/23. Kahlert CR. Clin Infect Dis.
- Effects of remdesivir in patients hospitalised with COVID-19: a systematic review and individual patient data meta-analysis of randomised controlled trials. 2/24/23. Amstutz A. Lancet Respir Med.
- Efficacy of awake prone positioning in patients with covid-19 related hypoxemic respiratory failure: systematic review and meta-analysis of randomized trials. 2/6/23. Weatherald J. BMJ.
Newsletter Issue 161, March 27, 2023:
- Risk of venous thromboembolism in non-respiratory and respiratory presentations of COVID-19 in critically ill patients. 2/14/23. Roubinian NH. Chest.
This research letter reports a retrospective cohort study of adult Kaiser Permanente patients who were PCR tested for SARS-CoV-2 before admission to 21 ICUs between December 1, 2020 and April 30, 2022. Authors assessed the incidence of venous thromboembolism (VTE) within 90 days of hospital admission, comparing those who presented with respiratory versus nonrespiratory diagnoses. Of these 11,143 cases, 5,440 (49%) were admitted with respiratory diagnoses. 2,983 (27%) had COVID-19, of whom 2,428 (81%) were admitted with respiratory diagnoses. ICU patients admitted with respiratory diagnoses has a higher 90-day incidence of VTE compared with those with nonrespiratory diagnoses (13.4% vs. 7.4%). SARS-CoV-2 infection was not associated with an increased risk of VTE among patients with nonrespiratory diagnoses, regardless of vaccination status. In contrast, the relative risk (RR) of VTE was increased in those admitted to ICU with respiratory diagnoses, including unvaccinated COVID-19 patients (RR 2.9), vaccinated COVID-19 patients (RR 2.0) and those without COVID-19 (RR 1.3). The increased risk of VTE in unvaccinated and vaccinated COVID-19 patients persisted during the period of Omicron variant predominance.
SAB Comment: This dataset helps further characterize the risk of VTE for patients with COVID-19 based upon symptoms at presentation. This study may act as a basis for further studies to better target prophylaxis for VTE in critically ill COVID-19 patients. - Early Treatment with Pegylated Interferon Lambda for Covid-19. 2/13/23. Reis G. N Engl J Med.
In this randomized, controlled, adaptive platform, Phase III, outcome based trial, vaccinated adult outpatients who presented with COVID-19 acute respiratory symptoms within 7 days of onset were treated either with pegylated interferon lambda (n=931, single subcutaneous injection, 180 μg) or placebo (n=1,018). The primary outcome of a COVID-19-related emergency department visit or hospitalization within 28 days of randomization occurred in 2.7% following the drug vs. 5.6% following placebo (OR of 0.49). These authors concluded that early treatment with Interferon conveyed a > 50% reduction risk of ER visit or hospitalization, irrespective of vaccine status (0, 1, 2, or 3 shots) and was also effective against multiple variants (Alpha, Delta and Omicron). The authors noted an additional benefit in lowering the viral load. The benefit differed amongst SARS-CoV-2 variants with Omicron > Delta > Alpha.  Determinants of Professional Fulfillment and Burnout Among Intensivists: A National Survey by the Society of Critical Care Anesthesiologists in 2022. 2/15/23. Siddiqui S. Anesth Analg.
Determinants of Professional Fulfillment and Burnout Among Intensivists: A National Survey by the Society of Critical Care Anesthesiologists in 2022. 2/15/23. Siddiqui S. Anesth Analg.
This is a cross-sectional survey of Society of Critical Care Anesthesiologists members in ICU practices in the US using the Stanford Personal Fulfillment Index. Response rate was 29%, 175 of 606. Several factors were associated with higher levels of personal fulfillment (likely to be an inverse of “burnout”) including age >45 years, ≤15 weeks per year of full time ICU coverage, and nighttime on-call supervision from home rather than in hospital. Adequate staffing allowed the coverage from home and was deemed better for clinician well-being. A favorable public perception of intensivists may have contributed positively and eventually provided additional emotional reward. The survey was comprehensive and assessed personal fulfillment, work exhaustion and interpersonal disengagement. This study sheds light on important underlying causes of healthcare worker loss and reinforces the need for further study.- Infection-induced immunity is associated with protection against SARS-CoV-2 infection and decreased infectivity. 2/12/23. Frutos AM. Clin Infect Dis.
Investigators present data from an ongoing household cohort study between March 2020-November 2022 in Nicaragua to determine transmission after one household member tests positive for SARS-CoV-2. There were 2,399 active participants in the cohort with 87 new/re-enrollees, 394 withdrawn, and 27 deaths. Within this subsidy of SARS-CoV-2 transmission, a total of 228 households (51.9% of all cohort houses) were activated (some multiple times) with 349 total activations. Of activated household contacts, 81.5% consented to intensive monitoring. Transmission occurred in 70% of households, with 41% of household contacts becoming infected. The secondary attack risk ranged from 8% to 14% depending on the time period. Symptomatic infected individuals were more infectious (RR 21.2) and participants with a prior infection were half as likely to be infected compared to naïve individuals (RR 0.52). While young children were less infectious, neither prior infection nor asymptomatic presentation reduced their infectivity (as was seen for adults). Authors comment that as SARS-CoV-2 becomes endemic, children may become more important in transmission dynamics. - Past SARS-CoV-2 infection protection against re-infection: a systematic review and meta-analysis. 2/16/23. Stein M. Lancet.
This meta-analysis of 65 studies (up to September 31, 2022) provides a comprehensive review of the effectiveness of past infection on outcomes (infection, symptomatic disease and severe disease), variant, and time since previous infection. The analysis shows high levels of protection against reinfection from all pre-Omicron variants (82%), but significantly reduced protection against reinfection from Omicron BA.1 (42%). Levels of protection against severe disease remained high for all variants (pooled protection of 78%), including Omicron BA.1. When analyzed as a function of time since previous infection, pre-Omicron infections afforded initially high protection (85%) which waned to 79% at 40 weeks. By contrast, protection from the Omicron BA.1 variant declined more rapidly, decreasing to 36% at 40 weeks. Although protection from reinfection by all variants wanes over time, the level of protection afforded by previous infection is at least as high, if not higher, than that provided by two-dose vaccination using mRNA vaccines.
SAB Comment: The number of studies on vaccine efficacy far exceeds the number of studies on the protection against COVID-19 by previous infection, yet protection afforded by previous infection is likely to be at least as important as vaccination status, and could be factored into public health approaches to COVID-19. This study provides the information needed to address protection from previous infections, at least through Omicron BA.1. - Early Estimates of Bivalent mRNA Booster Dose Vaccine Effectiveness in Preventing Symptomatic SARS-CoV-2 Infection Attributable to Omicron BA.5- and XBB/XBB.1.5-Related Sublineages Among Immunocompetent Adults – Increasing Community Access to Testing Program, United States, December 2022-January 2023. 2/2/23. Link-Gelles R. MMWR Morb Mortal Wkly Rep.
This is a CDC report on the bivalent mRNA vaccine effectiveness (VE) against symptomatic infection by recent SARS-CoV-2 variants BA.5 and XBB/XBB1.5. Using data from the Increasing Community Access to Testing Program during December 1, 2022 to January 13, 2023, the authors used a test negative control format to evaluate 29,175 immunocompetent adult patients with COVID-19-like symptoms who had received two or more monovalent mRNA vaccine/booster doses. In persons 18-49 years of age who had additionally received a bivalent mRNA booster 2 to 3 months earlier compared with no bivalent booster, VE was 52% against symptomatic BA.5 infection and 48% against symptomatic XBB/XBB1.5 infection. Other age groups also had modest but real VE with a bivalent booster.
SAB Comment: As featured in two articles in Newsletter Issue 159 (“Early Estimates of Bivalent mRNA Vaccine Effectiveness in Preventing COVID-19-Associated Hospitalization Among Immunocompetent Adults Aged ≥65 Years – IVY Network, 18 States, September 8-November 30, 2022” and “Early Estimates of Bivalent mRNA Vaccine Effectiveness in Preventing COVID-19-Associated Emergency Department or Urgent Care Encounters and Hospitalizations Among Immunocompetent Adults – VISION Network, Nine States, September-November 2022“) bivalent boosters reduced the incidence of hospitalizations and urgent care or emergency department evaluations. This is the first evaluation of VE of the bivalent booster against symptomatic infections with the most recent BA.5 and XBB/XBB1.5 lineages and suggests that all persons should stay up to date with recommended COVID-19 vaccines. - Below you will find a list of additional articles and resources selected for the IARS COVID-19 Resource website, all of which include a summary with major takeaways and are searchable by topic and date posted on the website:
Newsletter Issue 160, February 27, 2023:
- Reports of Guillain-Barré Syndrome After COVID-19 Vaccination in the United States. 2/1/23. Abara WE. JAMA Netw Open.
Relying on the Vaccine Adverse Event Reporting System (VAERS), this CDC and FDA-authored retrospective cohort study analyzes the occurrence of Guillain-Barré Syndrome (GBS) within 21-42 days following 3 different COVID-19 vaccines administered between December 2020 and January 2022. Among 488 million vaccinations, there were 295 verified cases of GBS and a 9-12 times higher incidence following vaccination with Ad26.COV2.S (Janssen) compared to BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna). While contributing cause and associate risk factors remain unclear, the authors identify a number of potential mechanisms related to the immune response elicited by the vaccine on a molecular level. The Advisory Committee on Immunization Practices recommends the mRNA COVID-19 vaccine rather than Ad26.COV2.S when both are available but bases this recommendation primarily on the increased risk of developing thrombosis with thrombocytopenia syndrome in addition to the risk of developing GBS.  Long COVID: major findings, mechanisms and recommendations. 1/13/23. Davis HE. Nat Rev Microbiol.
Long COVID: major findings, mechanisms and recommendations. 1/13/23. Davis HE. Nat Rev Microbiol.
This literature review summarizes the current knowledge about most aspects of long COVID. Starting with epidemiology (an estimated incidence of 10-30% of nonhospitalized cases, 50-70% of hospitalized cases and 10-12% of vaccinated cases), the authors move through the known and postulated etiologies of long COVID, including immunologic dysregulation, reactivation of SARS-CoV-2 and other associated viruses, clotting and endothelial abnormalities, dysfunctional neurological signaling and other etiologies. Long COVID of the commonly affected organ systems is reviewed, as well as the diagnostic tools and treatments currently in use. Some of the “miscues” about long COVID are briefly mentioned. The impact of vaccination and re-infection on established long-COVID patients is discussed. Particular attention is paid to myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and dysautonomia, especially postural orthostatic tachycardia syndrome (POTS). The authors clearly state that much more research is needed to understand and treat long COVID.
SAB Comment: This exhaustive review packs a huge amount of information into just a few pages, with useful tables and graphs. Some of the authors are members of the Patient-Led Research Collaborative, a team of long-COVID patients with a wide range of research, policy, design and medical backgrounds.- Adherence to Healthy Lifestyle Prior to Infection and Risk of Post-COVID-19 Condition. 2/6/23. Wang S. JAMA Intern Med.
The role of a healthy lifestyle before infection in reducing post-COVID condition (PCC) was the focus of this substudy within the Nurses’ Health Study II, a prospective cohort which enrolled participants in 1989. The substudy enrolled 32,249 nurses for whom lifestyle data from 2017 was available. They completed monthly and quarterly surveys beginning in April 2020, reporting testing positive for SARS-CoV-2 (1,981), and the presence of symptoms for at least 4 weeks (871 or 44%). The 2017 lifestyle parameters (BMI, smoking, alcohol consumption, diet, physical activity and sleep) were individually and collectively compared between those with and without PCC. As the number of healthy lifestyle factors increased, the likelihood of PCC decreased; those with 5-6 healthy parameters had a 49% lower risk of PCC. The most significant factors were BMI (risk ratio [RR] 0.85) and sleep (RR 0.83).
SAB Comment: This report contains a great deal of data, and includes 10 sensitivity analyses, as well as population attributable risk (PAR) information. The authors posit that the anti-inflammatory effect of a healthy lifestyle mitigates PCC. It must be kept in mind that the population was 97% white and limited to women aged 55-75, who were willing to share their health information for more than 30 years. - VV116 versus Nirmatrelvir-Ritonavir for Oral Treatment of Covid-19. 12/28/22. Cao Z. N Engl J Med.
VV116 is an orally effective antiviral remdesivir analogue developed in China and undergoing multicenter, observer blinded, randomized controlled trial on symptomatic patients at high risk for progression to severe COVID-19. To compare duration of symptoms and time to clinical recovery, SARS-CoV-2 infected patients with a median age of 53 and mostly vaccinated, were recruited from 7 Shanghai hospitals during the month of April 2022 and assigned to receive either the study drug (n=384) or nirmatrelvir-rotinavir (Paxlovid) (n=387) orally twice a day. The result showed that among symptomatic adults hospitalized with mild to-moderate COVID-19 with risk factors including age older than 50 years, cardiac disease and obesity, a 5-day course of oral treatment with VV116 was noninferior to nirmatrelvir–ritonavir in shortening the time to sustained clinical recovery (HR 1.17, [CI 1.01 to 1.35]). Among the limitations listed in this industry-funded study is the inability to draw conclusions about the efficacy of VV116 for the prevention of progression to severe or critical COVID-19 or death, because no events occurred in either group.
SAB Comment: This is one of several recent studies exploring oral antiviral alternatives to Paxlovid and remdesivir. Worldwide shortages, high costs and the need for parenteral administration of remdesivir are of concern for currently approved antivirals, as well as a high percentage of contraindications due to impaired renal and hepatic function and drug-drug interactions among patients who would otherwise be prime candidates for these treatments based on their risk category. - Physical interventions to interrupt or reduce the spread of respiratory viruses. 1/30/23. Jefferson T. Cochrane Reviews.
This Cochrane Review metanalysis has generated significant press controversy despite the clearly stated concerns of its authors that more and better studies are essential to gaining an adequate understanding of the effects of these interventions. This review is an update of one last published in 2020 and only 2 of the 9 studies used to develop the updated first and primary conclusion concerned COVID-19 infection. That conclusion was that wearing masks in the community probably makes little or no difference to the outcome of influenza‐like illness or COVID‐19 like illness compared to not wearing masks (risk ratio (RR) 0.95, 9 trials, 276,917 participants); or in the outcome of laboratory‐confirmed influenza/SARS‐CoV‐2 (RR 1.01, 6 trials, 13,919 participants). However, it’s worth noting that both COVID-19 studies used were designed to test a mask encouraging strategy, not mask wearing, and both showed a small decrease in infection rate in the intervention group which was statistically significant in the larger of the two studies (but not the smaller).
Two additional conclusions are presented: 1) The use of a N95/P2 respirators compared to medical/surgical masks probably makes little or no difference for the objective outcome of laboratory‐confirmed influenza infection (RR 1.10, 5 trials, 8407 participants), and 2) Pooled data showed that hand hygiene may be beneficial with an 11% relative reduction of respiratory illness (RR 0.89, low‐certainty evidence with high heterogeneity).
The authors state that, “The high risk of bias in the trials, variation in outcome measurement, and relatively low adherence with the interventions during the studies hampers drawing firm conclusions.”
SAB Comment: We’re impressed, as are the authors, that there are so few randomized controlled trials published to date of the effectiveness of mask wearing during the COVID-19 epidemic but wonder, given the lethality of the virus, whether researchers have been hesitant to define a control group. - Below you will find a list of additional articles and resources selected for the IARS COVID-19 Resource website, all of which include a summary with major takeaways and are searchable by topic and date posted on the website:
- Vaccination status and long COVID symptoms in patients discharged from hospital. 2/11/23. Nascimento TCDC. Sci Rep.
- Long covid outcomes at one year after mild SARS-CoV-2 infection: nationwide cohort study. 1/11/23. Mizrahi B. BMJ.
- Beyond acute COVID-19: A review of long-term cardiovascular outcomes. 2/8/23. Parhizgar P. Can J Cardiol.
- Cardiologic Manifestations in Omicron-Type Versus Wild-Type COVID-19: A Systematic Echocardiographic Study. 1/25/23. Ghantous E. J Am Heart Assoc.
Newsletter Issue 159, January 23, 2023:
- SAB Comment for the following three studies summarized below: The two well-done clinical studies provide much anticipated bivalent vaccine effectiveness data as Omicron variants continue to evolve and many infections are uncounted. Despite lab data that suggest viral neutralization after the bivalent vaccine is poor (lab study below titled “Alarming Antibody Evasion…), these clinical studies document real but moderate protection against visits to urgent care or emergency departments and hospitalization. This protection seems to be most prominent in older adults and the time since the previous monovalent vaccine dose was of greater significance. These studies do not address the durability of bivalent booster effectiveness. This information suggests that all eligible persons should stay up to date with recommended COVID-19 vaccinations despite indications that we may be headed for a period of increased breakthrough infections.
- Early Estimates of Bivalent mRNA Vaccine Effectiveness in Preventing COVID-19-Associated Emergency Department or Urgent Care Encounters and Hospitalizations Among Immunocompetent Adults – VISION Network, Nine States, September-November 2022. 12/29/22. Tenforde MW. MMWR Morb Mortal Wkly Rep.
This is a bivalent vaccine effectiveness (VE) study from the VISION Network (nine US states) during September 13 to November 18, 2022, specifically looking at effectiveness against Emergency Department (ED) and Urgent Care (UC) encounters as well as hospitalizations. Among 78,303 ED/UC encounters in immunocompetent adults (over 18 years old), VE of the bivalent vaccine was 56% compared to no vaccination, 31% compared with receipt of last monovalent dose 2-4 months earlier, and 50% compared with receipt of last monovalent dose 11 months or more earlier. Among 15,527 hospitalizations of immunocompetent adults, the booster VE was similar to that in ED/UC patients. - Early Estimates of Bivalent mRNA Vaccine Effectiveness in Preventing COVID-19-Associated Hospitalization Among Immunocompetent Adults Aged ≥65 Years – IVY Network, 18 States, September 8-November 30, 2022. 12/29/22. Surie D. MMWR Morb Mortal Wkly Rep.
These IVY Network investigators from 18 US states sought to define vaccine effectiveness of the bivalent COVID-19 vaccine against hospitalization in elderly patients. Between September 8 and November 30, 2022, 798 patients hospitalized with a COVID-like illness were enrolled in this test-negative analysis. Among immunocompetent adults aged ≥65 years, a bivalent booster dose received after ≥2 monovalent mRNA doses provided good protection (73%) against COVID-19-associated hospitalization during this period of Omicron BA.5 or BQ.1/BQ.1.1 predominance. Compared to unvaccinated patients, the bivalent vaccine effectiveness was 84%. Substantial additional protection from a bivalent booster dose was observed when compared with remote monovalent-only mRNA vaccination. - Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants. 12/29/22. Wang Q. Cell.
This is a detailed in-vitro study of antibody resistance and viral receptor binding affinity of SARS-CoV-2 Omicron BQ.1, BQ.1.1, XBB, and XBB.1 subvariants, that are now prevalent and rapidly expanding globally. Sera from 5 cohorts (n=14-21 each) with the following histories were tested: 1) 3 doses of an original COVID-19 mRNA vaccine, 2) 4 doses of an original COVID-19 mRNA vaccine, 3) a fourth vaccination with a bivalent COVID-19 mRNA vaccine, 4) breakthrough BA.2 infection post-vaccination, and 5) breakthrough BA.4 or BA.5 infection. Results showed BQ.1.1 is approximately 6-fold more resistant to serum neutralization than its predecessor BA.5. XBB.1 is ∼63-fold more resistant to serum neutralization than its predecessor, or ∼49-fold more resistant than BA.4/5. SARS-CoV-2 breakthrough infection induced somewhat better antibody responses than vaccination. In addition, the clinically authorized monoclonal antibodies bebtelovimab and Evusheld could not neutralize BQ or XBB variants, leaving no effective preventative treatments for immunocompromised patients. The publication includes illustrative graphs, tables, and structural analysis. Analysis of cellular immunity and clinical studies are called for; however previous in-vitro studies have been predictive of in vivo outcomes.
- Early Estimates of Bivalent mRNA Vaccine Effectiveness in Preventing COVID-19-Associated Emergency Department or Urgent Care Encounters and Hospitalizations Among Immunocompetent Adults – VISION Network, Nine States, September-November 2022. 12/29/22. Tenforde MW. MMWR Morb Mortal Wkly Rep.
- SAB Comment for the following two articles summarized below: The two studies below are among those that assess the real-world effect of Paxlovid during the Omicron era. They show similar reductions in hospitalization rate which are not nearly as large as that seen in the Paxlovid phase 2-3 trial done with unvaccinated patients during the Delta variant surge in 2021 (87.8% reduction of combined death or hospitalization). In these recent studies the rates of hospitalization or mortality are significantly reduced in all patients (Paxlovid treated or untreated) compared to the original phase 2-3 study likely related to a combination of vaccinations, prior infections and decreasing virulence of evolving viral strains. It’s interesting that as a consequence, both of the studies below show that the number of patients needed to treat to achieve a single improved outcome (NNT) in hospitalization or combined hospitalization/mortality exceeds 200 patients.
 Paxlovid Associated with Decreased Hospitalization Rate Among Adults with COVID-19 — United States, April–September 2022. 12/2/22. Shah M. MMWR Morb Mortal Wkly Rep.
Paxlovid Associated with Decreased Hospitalization Rate Among Adults with COVID-19 — United States, April–September 2022. 12/2/22. Shah M. MMWR Morb Mortal Wkly Rep.
This study reviewed electronic medical records to show that of 699,848 adults aged ≥18 years who were eligible for Paxlovid during April-August 2022, 28.4% received a Paxlovid prescription within 5 days of COVID-19 diagnosis (of whom 68.8% previously had received two or more doses of mRNA vaccine). Being prescribed Paxlovid was associated with a lower hospitalization rate among the overall study population (adjusted hazard ratio [aHR] = 0.49). The reduction was similar among those who had received ≥3 mRNA COVID-19 vaccines (aHR = 0.50) and across all age groups. The proportion of persons with in-hospital death was also lower among persons who received Paxlovid (0.01%) than among those who did not (0.04%). The authors state that Paxlovid should be prescribed to eligible adults to reduce the risk of COVID-19-associated hospitalization.
SAB Comment: This study shows a significant real-world effect of Paxlovid during the Omicron era but with a reduction in hospitalization rate that is not nearly as large as that seen in the Paxlovid phase 2-3 trial done with unvaccinated patients during the Delta variant surge in 2021 (87.8% reduction of combined death or hospitalization).- Nirmatrelvir Plus Ritonavir for Early COVID-19 in a Large U.S. Health System : A Population-Based Cohort Study. 12/12/22. Dryden-Peterson S. Ann Intern Med.
This study was designed to assess the real-world efficacy of nirmatrelvir plus ritonavir (Paxlovid) for early outpatient COVID-19 during the first 6 months of 2022 in an era of prevalent SARS-CoV-2 immunity and immune-evasive Omicron subvariants. It attempts to emulate a clinical trial using observational data from 117,000 patients diagnosed with COVID-19 to determine the drug combination’s efficacy in preventing hospitalization within 2 weeks of diagnosis and death of any causes within 28 days. Of 44,000 outpatients receiving Paxlovid, 69 (0.55%) were hospitalized compared to 310 (.97%) among the 32,000 patients who were not, resulting in an adjusted risk ratio for Paxlovid of 0.56 (CI, 0.42-0.75), confirming the drug combination’s effectiveness among vaccinated and unvaccinated patients aged 50 and older.
An accompanying editorial compliments the author’s attempt to minimize bias in a retrospective data evaluation, lends perspective to the value of recent trials and reviews current literature.
SAB Comment: This is a high-profile retrospective real-world study corroborating Paxlovid’s effectiveness.
- Prevalence of Contraindications to Nirmatrelvir-Ritonavir Among Hospitalized Patients With COVID-19 at Risk for Progression to Severe Disease. 11/15/22. Hoertel N. JAMA Netw Open.
Nirmatrelvir-ritonavir (Paxlovid) reduces COVID-related morbidity and mortality. Hospitalized patients may have contraindications to its use. Ritonavir may elevate drug concentrations if dependent on hepatic cytochrome P-450-3A (CYP3A) metabolism. Coadministration with CYP3A inducers can significantly reduce nirmatrelvir concentrations with loss of drug response. Severe kidney and liver disease patients were excluded from clinical trials. In 36 Parisian University Hospitals, the authors studied records from January 24, 2020 to November 30, 2021, a period before patients received nirmatrelvir-ritonavir. Of 62,500 PCR-proven patients; 9,136 (14.6%) had a medical contraindication (men 18%, women 11%; older (>65)- 27%; co-morbidities-37%). Among 4,861 patients who died, 50.7% had a contraindication. The authors conclude that published nirmatrelvir-ritonavir hospital studies may overestimate treatment efficacy by avoiding patients with contraindications. A large unmet need remains for effective COVID treatments. - Diaphragm Muscle Weakness Might Explain Exertional Dyspnea Fifteen Months After Hospitalization for COVID-19. 1/3/23. Regmi B. Am J Respir Crit Care Med.
The etiology of long COVID/Post-COVID Condition (PCC) remains an enigma. Recent studies consider multiple causes which remain under investigation. Additional questions remain whether invasive ventilation increases the incidence or severity of symptoms. Many COVID-19 survivors report dyspnea that cannot be explained by routine clinical diagnostic measures, including pulmonary function tests and cardiac evaluation. This study investigated 50 patients previously hospitalized with COVID-19 (14 female, age 58±12 years), half were treated with mechanical ventilation and the remaining outpatients using pulmonary function testing, 6-minute walk test, echocardiography, twitch transdiaphragmatic pressure following cervical magnetic stimulation of the phrenic nerve roots, and diaphragm ultrasound. Diaphragm function data were compared with values from a healthy control group. Testing evaluated both voluntary and involuntary (magnetic phrenic nerve stimulation) measurements of inspiratory and expiratory power at FRC. Dyspnea was equally experienced between survivors who required invasive ventilation (including ECMO) and those managed without. Diaphragmatic inspiratory strength reflected in voluntary and magnetically induced twitch pressures generated at functional residual capacity (FRC) and diaphragmatic thickening noted on ultrasound were reduced. Expiratory values similarly measured showed minimal disruption of expiration. The study demonstrated diaphragm muscle weakness underlying otherwise unexplained exertional dyspnea in patients previously hospitalized for COVID-19 and suggested specific interventions, such as inspiratory muscle training, could be an effective approach to mitigate COVID-19 exertional dyspnea. - Two masks can be worse than one: N95 respirator failure caused by an overlying face mask. 12/20/22. Mueller JT. Infection Control & Hospital Epidemiology.
Adding an external procedure mask in front of a filtering face-piece respirator (FFR), such as an N95, has been suggested at times, largely to protect the FFR from soiling and to extend its use. One hundred healthcare workers from Mayo Clinic in Arizona, who successfully passed quantitative fit testing of an N95 respirator (3M 1870 Aura FFR), were immediately re-evaluated to assess the effect of adding a procedure mask (Halyard 47117) in front of their N95. Thirteen out of 100 participants failed the quantitative fit test following the addition of the procedure mask. Authors review potential causes as well as limitations of the study with regard to testing a single combination of face protection devices. Guidance on the use of N95 FFRs should consider the potential risk of increased fit failure when they are worn with an overlying face mask. Further research including other FFR + face-mask combinations is needed. - Optimized ACE2 decoys neutralize antibody-resistant SARS-CoV-2 variants through functional receptor mimicry and treat infection in vivo. 12/7/22. Torchia JA. Science Advances.
These investigators report the development of recombinant soluble forms of the cell surface ACE-2 protein to act as a therapeutic decoy receptor. The decoys show no loss of potency with recent variants. The decoy receptors engage the viral receptor-binding domain (RBD) and are thought to outcompete cell-surface ACE2 for viral binding. The authors state the decoy is in theory, identical to the cell-surface receptor. Therefore, viral mutations which would weaken binding to the decoy would weaken binding to the cell surface receptor and reduce infectivity. The optimized ACE2 decoy triggers irreversible structural changes in the critical viral RBD S-protein. These studies show how ACE2 decoys function and support therapeutic development for emerging ACE-2-dependent coronaviruses. - Below you will find a list of additional articles and resources selected for the IARS COVID-19 Resource website, all of which include a summary with major takeaways and are searchable by topic and date posted on the website:
- Association of Time to Surgery After COVID-19 Infection With Risk of Postoperative Cardiovascular Morbidity. 12/14/22. Bryant JM. JAMA Netw Open.
- Impact of SARS-CoV-2 variants on inpatient clinical outcome. 12/18/22. Robinson ML. Clin Infect Dis.
- Imprinted SARS-CoV-2 humoral immunity induces convergent Omicron RBD evolution. 1/3/23. Cao Y. Nature.
- Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial. 12/25/22. Butler CC. Lancet.
Best of 2022 Newsletter
As we begin this new year, we would like to reflect on all that has been accomplished with the IARS COVID-19 Resource Initiative as well as highlight some of the trending articles and CME activities that were featured in 2022. Now more than two years since this initiative was launched, the IARS COVID-19 Scientific Advisory Board remains dedicated to this important effort and continues to screen the peer-reviewed literature to identify and disseminate the most pertinent information to help frontline professionals navigate this ever-evolving pandemic via this newsletter and a resource website. At this current stage of the pandemic, articles relevant for clinical care for anesthesiologists and intensivists have begun to decrease. In response to this change, we will now deliver the COVID-19 Newsletter on a monthly basis to ensure each issue continues to provide the most critical information. As this pandemic continues to adapt so will this initiative. Learn more below.
 Wellness and Coping of Physicians Who Worked in ICUs During the Pandemic: A Multicenter Cross-Sectional North American Survey. 10/27/22. Burns KEA. Crit Care.
Wellness and Coping of Physicians Who Worked in ICUs During the Pandemic: A Multicenter Cross-Sectional North American Survey. 10/27/22. Burns KEA. Crit Care.
This survey supported by the American Thoracic Society assessed ICU attending physician wellness and coping during the COVID-19 pandemic. The results reflect a 43% response rate to 1,080 questionnaires, using four validated instruments from physicians in 62 US and Canadian sites, conducted February-April 2021. About 60% reported moral distress (conflict with internal ethical values) and burnout (emotional exhaustion, depersonalization, reduced personal accomplishment). Moral distress, correlated with increased workload (patient volume, days worked, unscheduled in-house night shifts), was exacerbated by adverse institutional organization, was greater in women and physicians of color, and contributed to a desire to leave their position in a quarter of respondents. Despite this, most physicians experienced moderate professional fulfillment (satisfaction from attaining career goals). The survey revealed four coping profiles: active planning/social interaction (20%), avoidance/self-distraction (45%), mixed (5%) or infrequent (30%).
SAB Comment: The phenomenon of pandemic-induced healthcare provider burnout is widely understood, but this survey provides new information on the extent and consequence of moral distress among intensivists. It also quantifies different work stress coping mechanisms, although ironically those who used them infrequently had less moral distress and burnout and more professional fulfilment. This group was older and could represent less intense “front-line” activity, a question not addressed by the authors. This point highlights that this survey quantitates problems but merely hints at solutions. Nonetheless, it provides an important database for future studies on interventions to ameliorate moral distress, burnout and work changes among intensivists.- The Next Next Wave: How Critical Care Might Learn From COVID in Responding to the Next Pandemic. 11/1/22. Tung A. Anesth Analg.
The Open Mind is an Anesthesia & Analgesia forum for “…proposing new approaches or solutions to an important issue facing the anesthesiology community.” In this article, “front-line” anesthesiology intensivists were asked how the critical care response to the next pandemic might be informed by their experience during COVID-19. Each section reflects provider experience at different institutions as follows: (1) managing hospital-wide critical care response to a new disease (NYU Langone Health); (2) converting operating rooms to ICUs (New York Presbyterian-Columbia); (3) evolving airway management of the COVID patient (University of Chicago); (4) adapting extracorporeal membrane oxygenation to COVID-associated acute respiratory failure (Emory University) and (5) the impact of COVID on provider wellness (Beth Israel Deaconess). Lessons learned include that the rapid, unpredictable evolution of pathogens requires nimble adaptability, collaboration and discovery; that published clinical guidance may lag behind empiric adaptation; and that provider stress must be recognized and supported.
SAB Comment: This is a unique overview based on the first-hand experience and contributions of anesthesiology intensivists over the almost three years of the COVID-19 pandemic. Given the pandemic’s enormous physical, emotional, economic and political toll, the lessons learned are germane to all intensivists, regardless of specialty, as well as hospital administrators and leadership. Readers are encouraged to also review the superb overview provided by the accompanying editorial. - Long-term neurological sequelae of SARS-CoV-2 infection. 10/3/22. Nat Med.
This is a research briefing by the senior author of a number of studies examining long-term sequelae of COVID-19 using the US Department of Veteran Affairs database. It highlights the fact that patients who survive the first 30 days of acute SARS-CoV-2 infection have a 42% increased risk of developing various neurological disorders during the subsequent year compared with uninfected contemporaries. Although the absolute burden of developing any neurological disorder might seem small (7% at 1 year), given the large scale of the pandemic, this translates into a large number of affected individuals. The authors used a cohort of 154,068 infected patients and 5,638,795 uninfected controls in addition to a prepandemic historical control of almost 6 million patients. Risk correlated with acute COVID severity. They emphasize the need for early recognition and treatment instead of dismissal of nonspecific symptoms, provide a number of references, and urge further investigation.
SAB Comment: A more detailed account of this research can be found below. Long-term neurologic outcomes of COVID-19. 9/22/22. Xu E. Nat Med.
Long-term neurologic outcomes of COVID-19. 9/22/22. Xu E. Nat Med. - COVID-19 and Acute Neurologic Complications in Children. 8/11/22. Antoon JW. Pediatrics.
This study is based upon data provided to the Pediatric Health Information System database from 52 pediatric academic centers during a 2-year period beginning March 2020. It focused on prevalence, risk factors and outcomes associated with neurological complications in hospitalized children. Among 15,137 children aged 2 months to 18 years, the overall incidence of acute neurological complication was 7%. Febrile seizures, nonfebrile seizures, and encephalopathy combined accounted for 85% of neurologic complications. Identified risk factors included preexisting chronic neurologic conditions, older age, race, and ethnicity. Lower odds occurred during the period of Delta-variant dominance, and in patients treated with remdesivir and dexamethasone. Neurologic complications were associated with increased mortality (1.8% vs. 0.6%, p<0.001), ICU admission, longer hospitalization, and higher cost of care. These results are comparable to children hospitalized with influenza and other viral illnesses and emphasize the importance of COVID-19 immunization, especially in a high-risk population. - Long-term cardiovascular outcomes of COVID-19. 2/8/22. Xie Y. Nat Med.
In this Veteran’s Administration database study, COVID-19 survivors who survived 30 days or more after their first positive test, exhibited increased risks and 12-month burdens of cardiovascular diseases, including cerebrovascular disorders, dysrhythmias, inflammatory heart disease, ischemic heart disease, heart failure, thromboembolic disease and other cardiac disorders. Two key findings: (1) the risks and associated burdens were evident among those who were not hospitalized during the acute phase of the disease; and (2) complications and associated burdens were correlated with the severity of the acute phase of COVID-19. This finding suggests that care pathways of people who survived the acute episode of COVID-19 should include attention to cardiovascular health and disease. Excellent description of methods accompanied by helpful tables.
SAB Comment: This VA study has important implications for the risk of perioperative major adverse cardiac events (MACE) in COVID-19 survivors. In comparing more than 150k COVID-19 survivors with more than 11 million controls, the risk of postacute cardiovascular manifestations was significantly higher in patients who had an ICU admission or were hospitalized than for those who were not hospitalized. These factors should be taken into consideration in the preoperative cardiovascular workup of COVID-19 survivors subsequently presenting for moderate or high-risk surgery.  Use of Cardiopulmonary Exercise Testing to Evaluate Long COVID-19 Symptoms in Adults: A Systematic Review and Meta-analysis. 10/12/22. Durstenfeld MS. JAMA Netw Open.
Use of Cardiopulmonary Exercise Testing to Evaluate Long COVID-19 Symptoms in Adults: A Systematic Review and Meta-analysis. 10/12/22. Durstenfeld MS. JAMA Netw Open.
This systematic review and meta-analysis examines changes in cardiopulmonary exercise testing (CPET) for patients with long COVID (LC). A total of 38 studies were identified that performed CPET on 2,160 individuals 3 to 18 months after SARS-CoV-2 infection, including 1,228 with symptoms consistent with LC. Most studies were case series of individuals with LC or cross-sectional assessments within posthospitalization cohorts. Based on a meta-analysis of 9 studies that compared a total of 464 LC patients with prevalent symptoms to 359 others without, the mean peak V̇O₂ was lowered by 4.9 mL/kg/min in symptomatic individuals. While cardiopulmonary exercise testing is not readily available and there are limitations including selection bias, limited data sets and variability in definitions, this study represents a compilation of the present understanding in this field and helps identify future areas of research.- Timing of elective surgery and risk assessment after SARS-CoV-2 infection: an update: A multidisciplinary consensus statement on behalf of the Association of Anaesthetists, Centre for Perioperative Care, Federation of Surgical Specialty Associations, Royal College of Anaesthetists, Royal College of Surgeons of England. 2/23/22. El-Boghdadly K. Anaesthesia.
This multidisciplinary update of the guidelines for surgery after SARS-CoV-2 infection comes from several United Kingdom medical organizations, focusing on the Omicron variant. The authors emphasize there is no data yet available regarding surgery after Omicron, and the previous need to wait 7 weeks to avoid any increase in risk stands. Avoiding surgery during active infection, vaccination, preoperative prevention measures, exercise, and hospital prevention measures are emphasized. Risk assessment with a tool such as Surgical Outcome Risk Tool v2 (SORT-2) is recommended along with an instructive example of how to calculate individual patient risk. The possibility that use of local or regional anesthesia may lower risk compared with general anesthesia is also discussed.  Covid-19 Vaccination and the Timing of Surgery Following Covid-19 Infection. 7/15/22. Le S. Annals of Surgery.
Covid-19 Vaccination and the Timing of Surgery Following Covid-19 Infection. 7/15/22. Le S. Annals of Surgery.
Kaiser Permanente investigators reviewed 228,913 adult elective inpatient and outpatient surgeries, prepandemic and after vaccination availability, to assess whether vaccination status or type of anesthesia affected postoperative complication rates following SARS-CoV-2 infection. Postoperative emergency room visits and unscheduled hospitalizations were increased only for patients not fully vaccinated at the time of surgery, and if it occurred less than 30 days following a COVID diagnosis (n=373, adjusted HR 1.55). The increased risk was fully accounted for in those who had general anesthesia (adjusted HR 1.84). Risks were not elevated when surgery occurred more than 4 weeks following a COVID diagnosis. Authors note “Current guidelines recommend deferring elective surgery for at least 7 weeks after Covid-19 diagnosis among patients who are asymptomatic at the time of surgery.” They recommend further study and liberalizing guidelines for those fully vaccinated or for whom general anesthesia can be avoided.
SAB Comment: This article prompted SAB review of a key cited reference, summarized below, that analyzed data from unvaccinated patients undergoing major elective surgery earlier in the pandemic. It contributed to guidelines suggesting a 7-8 week preoperative delay following a positive PCR. SARS-2-CoV continues to evolve with multiple variants and the specter of long COVID-19, and the concomitant evolution of vaccines and therapeutics. As a consequence, decisions regarding timing of elective surgery following a COVID diagnosis are best individualized to each patient. We anticipate ongoing studies regarding the important question of how to assess and minimize perioperative risks following COVID. Extended prone positioning duration for COVID-19-related ARDS: benefits and detriments. 7/8/22. Walter T. Crit Care.
Extended prone positioning duration for COVID-19-related ARDS: benefits and detriments. 7/8/22. Walter T. Crit Care.
This retrospective observational study in France from March 2020-April 2021 evaluated 81 COVID-19 patients using a strategy, originally implemented for organizational and human resources purposes. It was based on extending the duration of proning sessions (PP) up to 48 hours in patients ventilated for COVID-19-related ARDS. The primary objective, the occurrence of pressure injury was observed in 26% of patients and was equivalent to patients who had PP sessions of shorter duration for non-COVID-19-related ARDS. The presence of skin injury, the most common complication of PP, correlated with cumulative duration of PP sessions and length of ICU stay, not the duration of each session. Extended PP significantly reduced staff viral exposure and workload, allowing for most position changes during the daytime. Longer proning sessions were also associated with continued improvements in ventilatory parameters over the extended time.- N95 respirators: quantitative fit test pass rates and usability and comfort assessment by health care workers. 5/29/22. Ng I. Medical Journal of Australia.
Healthcare workers (n = 2,161) at the Royal Melbourne Hospital underwent quantitative fit testing of N95 respirators per Australian Infection Control Expert Group protocol, each with at least three of four types: semi-rigid cup, flat-fold cup, duckbill, and three-panel flat-fold. Images are available here. The overall fit test pass rates were 65% for the semi-rigid cup respirators, 32% for the flat-fold respirator, 59% for the duckbill respirators, and 96% for the three-panel flat-fold respirator. Three hundred seventy-eight participants completed a detailed comfort and usability survey. Overall comfort and assessment ratings differed significantly by type. Median overall comfort and overall assessment values were highest for the three-panel flat-fold model and lowest for the semi-rigid cup model. These results may inform institutional procurement decision-makers as well as individuals who may not have access to quantitative testing to enhance respiratory protection. - Best COVID-19 Activities:
 Covid-19 Vaccination and the Timing of Surgery Following Covid-19 Infection. 7/15/22. Le S. Annals of Surgery.
Covid-19 Vaccination and the Timing of Surgery Following Covid-19 Infection. 7/15/22. Le S. Annals of Surgery. Wellness and Coping of Physicians Who Worked in ICUs During the Pandemic: A Multicenter Cross-Sectional North American Survey. 10/27/22. Burns KEA. Crit Care.
Wellness and Coping of Physicians Who Worked in ICUs During the Pandemic: A Multicenter Cross-Sectional North American Survey. 10/27/22. Burns KEA. Crit Care. Long-term neurologic outcomes of COVID-19. 9/22/22. Xu E. Nat Med.
Long-term neurologic outcomes of COVID-19. 9/22/22. Xu E. Nat Med. Use of Cardiopulmonary Exercise Testing to Evaluate Long COVID-19 Symptoms in Adults: A Systematic Review and Meta-analysis. 10/12/22. Durstenfeld MS. JAMA Netw Open.
Use of Cardiopulmonary Exercise Testing to Evaluate Long COVID-19 Symptoms in Adults: A Systematic Review and Meta-analysis. 10/12/22. Durstenfeld MS. JAMA Netw Open. Extended prone positioning duration for COVID-19-related ARDS: benefits and detriments. 7/8/22. Walter T. Crit Care.
Extended prone positioning duration for COVID-19-related ARDS: benefits and detriments. 7/8/22. Walter T. Crit Care.
Newsletter Issue 157, December 19, 2022:
 A population-based serological study of post-COVID syndrome prevalence and risk factors in children and adolescents. 11/29/22. Dumont R. Nat Commun.
A population-based serological study of post-COVID syndrome prevalence and risk factors in children and adolescents. 11/29/22. Dumont R. Nat Commun.
This report is from a Swiss population study of 1,034 children, aged 6 months to 17 years, who were tested for anti-SARS-CoV-2 N antibodies December 2021-February 2022. Pediatric vaccinations were not available to participants until early January 2022. Their parents filled in a questionnaire on symptoms compatible with post-COVID syndrome lasting over 12 weeks. Of the 1,034 children tested, 570 (55.1%) were seropositive. Overall, 24% has a prior test confirming SARS-CoV-2 infection, and 19% had had clinical symptoms consistent with COVID-19. Both were far more prevalent in the seropositive cohort, (by a factor of ~8x). The sex- and age-adjusted prevalence of children with persistent symptoms was 9.1% among seropositive children and 5.0% among seronegative children, with an adjusted prevalence difference (ΔaPrev) = 4.1%. Stratifying by age group, only those 12-17 years of age displayed a substantial risk of having post-COVID symptoms (ΔaPrev = 8.3%). Identified risk factors for post-COVID syndrome were older age, lower socioeconomic status and chronic health conditions, especially asthma.
SAB Comment: This study is useful in estimating the population’s prevalence of long COVID in young children (0%) and in adolescents (8.3%) of all those with positive serologies but does not estimate a symptomatic COVID-infected adolescent’s risk of long COVID (which may be significantly higher). Because pediatric vaccinations began in Switzerland in January 2022, the study could not assess the effect of prior vaccination on persistent symptoms, as not enough participants were vaccinated.- Incidence of Viral Rebound After Treatment With Nirmatrelvir-Ritonavir and Molnupiravir. 12/6/22. Wong GL. JAMA Netw Open.
To better understand the much-discussed rebound phenomena when COVID-19 patients receive oral antiviral medication (molnupiravir (Lagevrio) or nirmatrelvir-rotinavir (Paxlovid)), 12,629 adult COVID-19 inpatients in Hong Kong were followed with RT-PCR testing during the first three months of 2022. Serial cycle threshold (Ct) values were used to measure rebound, defined as a Ct value that increased to > 40, followed by a decrease to 40 or less. Viral rebound was uncommon, occurring in 0.6% of patients who did not receive an antiviral (n=11,688), 0.8% in molnupiravir patients (n=746), and 1% in nirmatrelvir-rotinavir patients (n=195). Viral rebound was not associated with an increased risk of mortality.
SAB Comment: It’s important to note that this study evaluates viral and not symptom rebound in hospitalized patients. Clinical symptoms were not studied. This study is similar to a research letter previously presented in newsletter 148. Both show a low incidence of viral rebound in treated as well as untreated patients. One study of the mechanism of rebound suggests that a more robust immune response rather than uncontrolled viral replication is the cause of clinical rebound. - Below you will find a list of additional articles and resources selected for the IARS COVID-19 Resource website, all of which include a summary with major takeaways and are searchable by topic and date posted on the website:
- Higher versus Lower Dose Corticosteroids for Severe to Critical COVID-19: A Systematic Review and Dose-Response Meta-analysis. 11/30/22. Pitre T. Ann Am Thorac Soc.
- Apparent risks of postural orthostatic tachycardia syndrome diagnoses after COVID-19 vaccination and SARS-Cov-2 Infection. 12/12/22. Kwan AC. Nature Cardiovascular Research.
Newsletter Issue 156, December 5, 2022:
- Trends in Clinician Burnout With Associated Mitigating and Aggravating Factors During the COVID-19 Pandemic. 11/23/22. Linzer M. JAMA Health Forum.
Presented are the results of 204 ongoing surveys regarding burnout administered to 56,000 physicians and advanced caregivers by members of the AMA partner healthcare systems, between February 2019 and December 2021, with 21,000 responses. Using the Mini Z work-life measure, topics included clinician outcomes (stress, satisfaction, burnout and intent to leave), and mitigating or aggregating factors (feeling valued, teamwork, work pace, work control, chaos, electronic health record factors, time pressure, and values alignment). Burnout occurred with 45% of respondents in 2019 and by late 2021, it was 60%. Intent to leave rose from 24% to greater than 40%. Mitigating factors that reduced burnout significantly were a calm vs chaotic environment 42%, good work control 36%, good teamwork 39% and feeling valued 32%. The authors concluded that monitoring and improving factors of providers’ working conditions may aid workforce retention.  Incidence of Epilepsy and Seizures Over the First 6 Months After a COVID-19 Diagnosis: A Retrospective Cohort Study. 11/17/22. Taquet M. Neurology.
Incidence of Epilepsy and Seizures Over the First 6 Months After a COVID-19 Diagnosis: A Retrospective Cohort Study. 11/17/22. Taquet M. Neurology.
To find the incidence of new-onset seizures and epilepsy after COVID-19 infection, these authors used data from a network of linked electronic health records that included over 81 million patients, mostly in the USA. Between January 2020 and May 2021, 152,754 patients with COVID-19 were compared to a matched cohort of 152,754 patients with influenza. The incidence of seizures within six months of COVID-19 was 0.81% (hazard ratio (HR) compared to influenza 1.55) and the incidence of epilepsy was 0.3% (HR compared to influenza 1.87). The incidence of epilepsy after COVID-19 compared to influenza was greater in people who had not been hospitalized and in individuals younger than 16 years old.
SAB Comment: An associated editorial discussed the clinical implications of these findings. Though the incidence of seizures or epilepsy is low, the large number of COVID-19 cases over the pandemic and the finding that milder cases are more at risk means that there may be many additional seizure and epilepsy patients presenting to providers after COVID-19. The etiology of the seizures and the long-term natural history for these patients is presently unknown.- Omicron sublineage BQ.1.1 resistance to monoclonal antibodies. 11/21/22. Arora P. Lancet Infect Dis.
This correspondence was submitted by a group of scientists working in a German primate center. They demonstrated on color charts how monoclonal antibodies (mAbs) suffer from increasing resistance and lack of effectiveness against several omicron sublineages. In particular, BQ.1.1, which is currently spreading in Europe and the US, is completely resistant to all mAbs in the authors’ model which consists of pseudovirus particles exposed to subclinical serum levels of a series of 16 mAbs, administered individually or in combination. Although this model awaits clinical confirmation, the findings indicate that novel, broadly active mAbs are urgently needed.
SAB Comment: The result of this lab study parallels clinical evidence of waning efficacy of monoclonal antibodies, including Evusheld, among patients exposed to omicron BQ.1.1 subvariants, currently responsible for roughly 50% of US infections. The study does not consider secondary resistance conferred by infection or vaccination. - Twice-Daily Oral Zinc in the Treatment of Patients With Coronavirus Disease 2019: A Randomized Double-Blind Controlled Trial. 11/11/22. Ben Abdallah S. Clin Infect Dis.
This prospective, randomized, double-blind, placebo-controlled, multicenter trial included both COVID-19 positive outpatients (n= 190) and inpatients (n= 280). Patients received 25mg of oral zinc (Zn) twice a day or placebo for 15 days. Mortality was 6.5% in the Zn group vs. 9.2% in the placebo group (OR 0.68, p=0.27, not statistically significant). ICU admission was 5.2% in the Zn group vs. 11.3% for the placebo group (OR 0.43. p=0.01). For inpatients, hospital length of stay was significantly shorter in the Zn group vs. the placebo group by 3.5 days. In outpatients, the duration of symptoms decreased by 1.9 days amongst the Zn treated group. Consistent results were observed in subgroups of patients based on age, comorbidities and oxygen therapy.
SAB Comment: The article didn’t mention serum Zn levels, so we cannot comment as to whether Zn prescription was for a Zn deficiency or used as a supplement. Larger studies should be done before Zn is considered a magic bullet for COVID-19 infection. - Below you will find a list of additional articles and resources selected for the IARS COVID-19 Resource website, all of which include a summary with major takeaways and are searchable by topic and date posted on the website:
- Cardiovascular outcomes after tixagevimab and cilgavimab use for pre-exposure prophylaxis against COVID-19: a population-based propensity-matched cohort study. 11/16/22. Birabaharan M. Clin Infect Dis.
- Platelet dysfunction and thrombus instability in flow conditions in patients with severe COVID-19. 11/14/22. Tacquard C. Thromb Res.
Newsletter Issue 155, November 21, 2022:
 Acute and postacute sequelae associated with SARS-CoV-2 reinfection. 11/10/22. Bowe B. Nat Med.
Acute and postacute sequelae associated with SARS-CoV-2 reinfection. 11/10/22. Bowe B. Nat Med.
This US Veterans Health Administration (VHA) study quantitates the health burdens of reinfection with SARS-CoV-2. Using the extensive VHA database, the outcomes for over 5 million people were compared between March 2020 and April 2022. The authors compared the cohorts with one infection (n=443,588), with two or more infections (n=40,947) and with no infection (n=5,334,729), during the acute illness and up to 6 months later. Compared to single infection, reinfection contributed additional risks of death (hazard ratio (HR) = 2.17), hospitalization (HR = 3.32) and sequelae in multiple organ systems. Evident regardless of vaccination status, these risks were most pronounced in the acute phase but persisted at six months. Compared to noninfected controls, cumulative risks and burdens of repeat infection increased according to the number of infections.
SAB Comment: This is a sobering picture of repeated infections. As variants evolve, immunity wanes and public health measures change, reinfection can result in major health problems, even for the vaccinated. Previous infection and vaccinations may reduce, but do not prevent COVID hospitalization, mortality and sequelae, including long COVID. The study is not designed to compare the outcomes of initial vs. subsequent infections, but it documents the potential seriousness of reinfection, and highlights the need for strategies to prevent reinfection.- Below you will find a list of additional articles and resources selected for the IARS COVID-19 Resource website, all of which include a summary with major takeaways and are searchable by topic and date posted on the website:
- Whole Health System Approach to Long COVID. 8/1/2022. Veterans Administration.
- Prevalence and Correlates of Long COVID Symptoms Among US Adults. 10/27/2022. Perlis RH. JAMA Netw Open.
- Variant-specific symptoms of COVID-19 in a study of 1,542,510 adults in England. 11/11/2022. Whitaker M. Nat Commun.
- Effect of vitamin D3 supplementation on cellular immunity and inflammatory markers in COVID-19 patients admitted to the ICU. 11/4/2022. Bychinin MV. Sci Rep.
- Association between vitamin D supplementation and COVID-19 infection and mortality. 11/13/2022. Gibbons JB. Sci Rep.
Newsletter Issue 154, November 14, 2022:
- Clinical phenotypes and outcomes associated with SARS-CoV-2 variant Omicron in critically ill French patients with COVID-19. 10/12/22. de Prost N. Nat Commun.
This prospective, multicenter observational study from France studied 259 patients with severe COVID-19 requiring ICU admission between December 6, 2021 and May 1, 2022. Extensive viral genomic sequencing was done and Delta and several early Omicron subvariants were identified. Mortality at 28 days in Delta and Omicron patients was not significantly different. The clinical phenotype of patients infected with Omicron was different from that in those infected with Delta. Omicron patients were older, frailer with more comorbidities (especially immunosuppression), and had higher severity of illness scores, reflecting more extrapulmonary organ failure. Among Omicron-infected patients, 43% were immunocompromised and these patients had a higher mortality compared with other Omicron-infected patients (47% vs 26%). Most immunocompromised Omicron-infected patients had been vaccinated (86%) but displayed a poor humoral response to vaccination.
SAB Comment: Continued deaths from COVID-19 during the Omicron period suggest that severe Omicron is a problem, even if general population studies suggest Omicron is not as severe as Delta. This study confirms that ICU patients have as poor a prognosis with Omicron as with Delta, and that the elderly, frail and immunocompromised population is particularly vulnerable. People with such risk factors may want to adopt strategies to avoid getting COVID-19 in the first place.  Effectiveness of Evusheld in Immunocompromised Patients: Propensity Score-Matched Analysis. 10/31/22. Najjar-Debbiny R. Science Trans Med.
Effectiveness of Evusheld in Immunocompromised Patients: Propensity Score-Matched Analysis. 10/31/22. Najjar-Debbiny R. Science Trans Med.
This is a real-world efficacy assessment of preexposure prophylactic intramuscular Evusheld administered to a cohort of immunosuppressed patients belonging to Israel’s largest healthcare system. On February 15, 2022, 703 patients were identified to be eligible and received 300 mg of the two-component monoclonal antibody (150 mg of tixagevimab and 150 mg of cilgavimab). This group was propensity matched with a 2,812 patient control group and followed through June 30 or up to 90 days focusing on COVID-19 related illness and hospitalization.
Results identified a reduced risk for SARS-CoV-2 infection and COVID-19-related hospitalization with a hazard ratio of 0.75 and 0.41, respectively. The reasons for the less promising results in this study compared to other recent studies were discussed. The need of a dosage increase, particularly for obese patients, was discussed along with an alert to expect lower responses as new variants emerge.
SAB Comment: This study parallels another previously reviewed paper highlighting Evusheld’s role in preexposure prophylaxis. In addition, a recent CDC fact sheet lists indication, timing and use for immunocompromised patients and those unable to be vaccinated. A higher 300 mg dose of each component (600 mg total) is to be repeated every 6 months where indicated and efficacy may decrease as variants evolve.- Discovery of SARS-CoV-2 antiviral synergy between remdesivir and approved drugs in human lung cells. 11/3/22. Nguyenla X. Sci Rep.
In this basic science study, these investigators conducted combinatorial high-throughput screening to find synergy of approved drugs with remdesivir to prevent SARS-CoV-2 replication in human lung epithelial cells. Of 20 approved drugs found, the strongest synergies were with the hepatitis C virus (HCV) NS5A inhibitors, velpatasvir and elbasvir, which may act on the SARS-CoV-2 exonuclease proofreader. These anti-HCV drugs are commercially available in combination with “partner” drugs, Epclusa® (velpatasvir/sofosbuvir) and Zepatier® (elbasvir/grazoprevir). When velpatasvir and elbasvir were combined with their “partner” drugs, it boosted remdesivir’s potency more than 25-fold. Further fine-tuning the potency and pharmacokinetic properties of these FDA-approved HCV therapeutics, used in combination with remdesivir, could merit clinical testing.
SAB Comment: As SARS-Co-V-2 continues to mutate, novel therapeutics will be crucial. In other viral illnesses (e.g., HIV), drug combinations yield benefits far beyond their constituents used alone. Unexpectedly, these investigators reported HCV drugs targeting a non-mutated part of the virus, the exonuclease proofreader, provide a remarkable boost to remdesivir concentrations achievable in vivo. However, much more work is needed before clinical trials can be considered. The in vitro concentrations required of the HCV drugs may not be achievable in patients and the expense of the HCV drugs may limit their usefulness. The authors also reported common generic drugs that appear to synergize with remdesivir in vitro. We await further testing. - Below you will find a list of additional articles and resources selected for the IARS COVID-19 Resource website, all of which include a summary with major takeaways and are searchable by topic and date posted on the website:
Newsletter Issue 153, November 7, 2022:
- The Next Next Wave: How Critical Care Might Learn From COVID in Responding to the Next Pandemic. 11/1/22. Tung A. Anesth Analg.
The Open Mind is an Anesthesia & Analgesia forum for “…proposing new approaches or solutions to an important issue facing the anesthesiology community.” In this article, “front-line” anesthesiology intensivists were asked how the critical care response to the next pandemic might be informed by their experience during COVID-19. Each section reflects provider experience at different institutions, as follows: (1) managing hospital-wide critical care response to a new disease (NYU Langone Health); (2) converting operating rooms to ICUs (New York Presbyterian-Columbia); (3) evolving airway management of the COVID patient (University of Chicago); (4) adapting extracorporeal membrane oxygenation to COVID-associated acute respiratory failure (Emory University) and (5) the impact of COVID on provider wellness (Beth Israel Deaconess). Lessons learned include that the rapid, unpredictable evolution of pathogens requires nimble adaptability, collaboration and discovery; that published clinical guidance may lag behind empiric adaptation; and that provider stress must be recognized and supported.
SAB Comment: This is a unique overview based on the first-hand experience and contributions of anesthesiology intensivists over the almost three years of the COVID-19 pandemic. Given the pandemic’s enormous physical, emotional, economic and political toll, the lessons learned are germane to all intensivists, regardless of specialty, as well as hospital administrators and leadership. Readers are encouraged to also review the superb overview provided by the accompanying editorial.  Wellness and Coping of Physicians Who Worked in ICUs During the Pandemic: A Multicenter Cross-Sectional North American Survey. 10/27/22. Burns KEA. Crit Care.
Wellness and Coping of Physicians Who Worked in ICUs During the Pandemic: A Multicenter Cross-Sectional North American Survey. 10/27/22. Burns KEA. Crit Care.
This survey supported by the American Thoracic Society assessed ICU attending physician wellness and coping during the COVID-19 pandemic. The results reflect a 43% response rate to 1,080 questionnaires, using four validated instruments from physicians in 62 US and Canadian sites, conducted February-April 2021. About 60% reported moral distress (conflict with internal ethical values) and burnout (emotional exhaustion, depersonalization, reduced personal accomplishment). Moral distress, correlated with increased workload (patient volume, days worked, unscheduled in-house night-shifts), was exacerbated by adverse institutional organization, was greater in women and physicians of color, and contributed to a desire to leave their position in a quarter of respondents. Despite this, most physicians experienced moderate professional fulfillment (satisfaction from attaining career goals). The survey revealed four coping profiles: active planning/social interaction (20%), avoidance/ self-distraction (45%), mixed (5%) or infrequent (30%).
SAB Comment: The phenomenon of pandemic-induced healthcare provider burnout is widely understood, but this survey provides new information on the extent and consequence of moral distress among intensivists. It also quantifies different work stress coping mechanisms, although ironically those who used them infrequently had less moral distress and burnout and more professional fulfilment. This group was older and could represent less intense “front-line” activity, a question not addressed by the authors. This point highlights that this survey quantitates problems but merely hints at solutions. Nonetheless, it provides an important database for future studies on interventions to ameliorate moral distress, burnout and work changes among intensivists.- Below you will find a list of additional articles and resources selected for the IARS COVID-19 Resource website, all of which include a summary with major takeaways and are searchable by topic and date posted on the website:
Newsletter Issue 152, October 24, 2022:
- Take advantage of COVID-19 Journal CME from newly published peer-reviewed articles from respected journals. The IARS COVID-19 Scientific Advisory Board (SAB) selects articles of the greatest clinical and scientific relevance to anesthesiologists, intensivists, related specialists and investigators. View the Journal CME First-Time User Guide for instructions.
 Use of Cardiopulmonary Exercise Testing to Evaluate Long COVID-19 Symptoms in Adults: A Systematic Review and Meta-analysis. 10/12/22. Durstenfeld MS. JAMA Netw Open.
Use of Cardiopulmonary Exercise Testing to Evaluate Long COVID-19 Symptoms in Adults: A Systematic Review and Meta-analysis. 10/12/22. Durstenfeld MS. JAMA Netw Open.
This systematic review and meta-analysis examines changes in cardiopulmonary exercise testing (CPET) for patients with long COVID (LC). A total of 38 studies were identified that performed CPET on 2,160 individuals 3 to 18 months after SARS-CoV-2 infection, including 1,228 with symptoms consistent with LC. Most studies were case series of individuals with LC or cross-sectional assessments within posthospitalization cohorts. Based on a meta-analysis of 9 studies that compared a total of 464 LC patients with prevalent symptoms to 359 others without, the mean peak V̇O₂ was lowered by 4.9 mL/kg/min in symptomatic individuals. While cardiopulmonary exercise testing is not readily available and there are limitations including selection bias, limited data sets and variability in definitions, this study represents a compilation of the present understanding in this field and helps identify future areas of research.
SAB Comment: As the importance of LC gains attention, this data summarizes prior literature and helps point the way forward. An accompanying podcast in the multimedia link summarizes the article well and adds to the clinical utility of these findings. Long-term neurological sequelae of SARS-CoV-2 infection. 10/3/22. Nat Med.
Long-term neurological sequelae of SARS-CoV-2 infection. 10/3/22. Nat Med.
This is a research briefing by the senior author of a number of studies examining long-term sequelae of COVID-19 using the US Department of Veteran Affairs database. It highlights the fact that patients who survive the first 30 days of acute SARS-CoV-2 infection have a 42% increased risk of developing various neurological disorders during the subsequent year compared with uninfected contemporaries. Although the absolute burden of developing any neurological disorder might seem small (7% at 1 year), given the large scale of the pandemic, this translates into a large number of affected individuals. The authors used a cohort of 154,068 infected patients and 5,638,795 uninfected controls in addition to a prepandemic historical control of almost 6 million patients. Risk correlated with acute COVID severity. They emphasize the need for early recognition and treatment instead of dismissal of nonspecific symptoms, provide a number of references, and urge further investigation.
SAB Comment: A more detailed account of this research can be found below.
Long-term neurologic outcomes of COVID-19. 9/22/22. Xu E. Nat Med. Circulating anti-nuclear autoantibodies in COVID-19 survivors predict long-COVID symptoms. 9/22/22. Son K. Eur Respir J.
Circulating anti-nuclear autoantibodies in COVID-19 survivors predict long-COVID symptoms. 9/22/22. Son K. Eur Respir J.
This longitudinal observational multi-institutional Canadian study of 106 COVID-19 patients from 8/2020 to 9/2021 examined the relationship between long COVID symptoms and antinuclear/extractable-nuclear antibodies (ANA/ENA). Patients, whose acute COVID symptoms varied, were evaluated at 3, 6, and 12 months with ANA/ENA and cytokine assays. They self-reported fatigue, coughing and dyspnea. Controls were age- and sex-matched, nonimmunized, COVID-negative volunteers (22), and non-COVID-19 positive patients with respiratory symptoms (34). At 3 months, ANA levels were increased in COVID patients compared to controls, but decreased over the year. Higher levels of tumor necrosis factor alpha, D-dimer and IL-1β predicted elevated ANA at 12 months. Tracking the increase of antibodies associated with autoimmune disease over time demonstrated potential de novo autoantibody synthesis. The authors conclude that persistently positive ANAs at 12 months post-COVID-19 are associated with continuing symptoms and inflammation in a subset of COVID survivors.
SAB Comment: The authors present elegant immunological evidence of an autoimmune factor in the development of long COVID. However, this small study has much potential for bias. Only 57 of 106 patients were available at 12 months. Also, in a study which concluded severity of disease was important, only 1 of 34 respiratory symptom control patients was hospitalized compared to 80 of 106 COVID-19 patients. Persistent circulating SARS-CoV-2 spike is associated with post-acute COVID-19 sequelae. 9/2/22. Swank Z. Clin Infect Dis.
Persistent circulating SARS-CoV-2 spike is associated with post-acute COVID-19 sequelae. 9/2/22. Swank Z. Clin Infect Dis.
This retrospective pilot study measured 3 SARS-CoV-2 antigens (using a Simoa assay) in blood samples from 63 individuals diagnosed with COVID-19, of whom 37 had postacute sequelae of COVID-19 (PASC) identified. SARS-CoV-2 spike antigen was detected several months postinfection in 60% of PASC and in none with COVID-19 only. Longitudinal blood samples were available in 12 PASC individuals and in 6 with COVID-19 only. Serial blood samples in 12 PASC individuals showed sustained or fluctuating spike antigen levels, whereas in samples of 6 with COVID-19 only, high antigen levels immediately after diagnosis dropped rapidly. The authors indicate that more study is needed and propose these results support the hypothesis that a reservoir of active virus persists within the body in individuals with PASC. Ten cytokines were also measured and were not different among the groups. The discussion further analyzes the validity of the findings.
Newsletter Issue 151, October 17, 2022:
 Use of Cardiopulmonary Exercise Testing to Evaluate Long COVID-19 Symptoms in Adults: A Systematic Review and Meta-analysis. 10/12/22. Durstenfeld MS. JAMA Netw Open.
Use of Cardiopulmonary Exercise Testing to Evaluate Long COVID-19 Symptoms in Adults: A Systematic Review and Meta-analysis. 10/12/22. Durstenfeld MS. JAMA Netw Open.
This systematic review and meta-analysis examines changes in cardiopulmonary exercise testing (CPET) for patients with long COVID (LC). A total of 38 studies were identified that performed CPET on 2,160 individuals 3 to 18 months after SARS-CoV-2 infection, including 1,228 with symptoms consistent with LC. Most studies were case series of individuals with LC or cross-sectional assessments within posthospitalization cohorts. Based on a meta-analysis of 9 studies that compared a total of 464 LC patients with prevalent symptoms to 359 others without, the mean peak V̇O₂ was lowered by 4.9 mL/kg/min in symptomatic individuals. While cardiopulmonary exercise testing is not readily available and there are limitations including selection bias, limited data sets and variability in definitions, this study represents a compilation of the present understanding in this field and helps identify future areas of research.
SAB Comment: As the importance of LC gains attention, this data summarizes prior literature and helps point the way forward. An accompanying podcast in the multimedia link summarizes the article well and adds to the clinical utility of these findings.- SARS-CoV-2 infection drives an inflammatory response in human adipose tissue through infection of adipocytes and macrophages. 9/22/22. MARTÍNEZ-COLÓN GJ. Science Trans Med.
Why obesity worsens COVID-19 outcomes is unknown. In COVID-19 autopsies, these investigators found SARS-CoV-2 RNA in epicardial, visceral, and subcutaneous adipocytes and in a subset of inflammatory adipose tissue-resident macrophages. Viral replication occurred in mature adipocytes, but adipose tissue-infiltrating macrophages synthesized viral components without producing infective virus (“abortive infection”). SARS-CoV-2 initiated an inflammatory response within both the infected macrophages and in bystander pre-adipocytes. Neither adipocytes nor macrophages consistently expresses ACE2 protein, suggesting viral entry by alternative mechanisms. The authors suggest that adipose tissue SARS-CoV-2 infection and induction of local and systemic responses driven by adipose tissue-resident macrophages could contribute to COVID-19 severity.
SAB Comment: This study represents early research that may lead to a better understanding of whether or not processes within adipocytes add to the expected pulmonary compromise of obesity during COVID. Future research is required. - Below you will find a list of additional articles and resources selected for the IARS COVID-19 Resource website, all of which include a summary with major takeaways and are searchable by topic on the website:
- Peripartum Outcomes Associated With COVID-19 Vaccination During Pregnancy: A Systematic Review and Meta-analysis. 10/3/22. Watanabe A. JAMA Pediatr.
- Pregnancy outcomes after SARS-CoV-2 infection in periods dominated by delta and omicron variants in Scotland: a population-based cohort study. 10/10/22. Stock SJ. Lancet Respir Med.
- Tracheostomy outcomes in critically ill patients with COVID-19: a systematic review, meta-analysis, and meta-regression. 10/1/22. Battaglini D. Br J Anaesth.
- Real-world effectiveness of molnupiravir and nirmatrelvir plus ritonavir against mortality, hospitalisation, and in-hospital outcomes among community-dwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the omicron wave in Hong Kong: an observational study. 10/10/22. Wong CKH. Lancet.
Newsletter Issue 150, October 12, 2022:
 Long-term neurological sequelae of SARS-CoV-2 infection. 10/3/22. Nat Med.
Long-term neurological sequelae of SARS-CoV-2 infection. 10/3/22. Nat Med.
This is a research briefing by the senior author of a number of studies examining long-term sequelae of COVID-19 using the US Department of Veteran Affairs database. It highlights the fact that patients who survive the first 30 days of acute SARS-CoV-2 infection have a 42% increased risk of developing various neurological disorders during the subsequent year compared with uninfected contemporaries. Although the absolute burden of developing any neurological disorder might seem small (7% at 1 year), given the large scale of the pandemic, this translates into a large number of affected individuals. The authors used a cohort of 154,068 infected patients and 5,638,795 uninfected controls in addition to a prepandemic historical control of almost 6 million patients. Risk correlated with acute COVID severity. They emphasize the need for early recognition and treatment instead of dismissal of nonspecific symptoms, provide a number of references, and urge further investigation.
SAB Comment: A more detailed account of this research can be found below.
Long-term neurologic outcomes of COVID-19. 9/22/22. Xu E. Nat Med.- Clinical, Virologic, and Immunologic Evaluation of Symptomatic Coronavirus Disease 2019 Rebound Following Nirmatrelvir/Ritonavir Treatment. 10/6/22. Epling BP. Clin Infect Dis.
This is a highly sophisticated analysis of the post-Paxlovid rebound phenomenon and its clinical significance. NIH investigators used viral sequencing and culture, serologic data, T-cell stimulation assays and soluble biomarkers in an 8-patient cohort of mostly Omicron BA.2 breakthrough infections who exhibited rebound and a 7-patient infected control group who did not.
Robust cytokine-producing, proliferating, activated SARS-CoV-2–specific T-cell responses were greater during rebound than during early acute COVID-19, along with rising T-cell counts. Anti-nucleocapsid IgG and Omicron-specific neutralizing antibodies were also increased in 6 patients with rebound symptoms.
This suggests that clinical rebound may be an indicator of a robust immune response rather than uncontrolled viral replication driving inflammation or a significant risk of impending disease progression. No patient developed severe disease during rebound, and adaptive immunity against SARS-CoV-2 appeared intact. - Below you will find a list of additional articles and resources selected for the IARS COVID-19 Resource website, all of which include a summary with major takeaways and are searchable by topic on the website:
- Clinical Features and Burden of Postacute Sequelae of SARS-CoV-2 Infection in Children and Adolescents. 10/4/22. Rao S. JAMA.
- Waning of vaccine effectiveness against moderate and severe covid-19 among adults in the US from the VISION network: test negative, case-control study. 10/3/22. Ferdinands JM. BMJ.
- Protection against omicron (B.1.1.529) BA.2 reinfection conferred by primary omicron BA.1 or pre-omicron SARS-CoV-2 infection among health-care workers with and without mRNA vaccination: a test-negative case-control study. 9/24/22. Carazo S. Lancet Infect Dis.
- Effectiveness of Molnupiravir in High Risk Patients: a Propensity Score Matched Analysis. 9/21/22. Najjar-Debbiny R. Clin Infect Dis.
- Correlation between COVID-19 severity and previous exposure of patients to Borrelia spp. 9/24/22. Szewczyk-Dąbrowska A. Sci Rep.
Newsletter Issue 149, October 5, 2022:
 Circulating anti-nuclear autoantibodies in COVID-19 survivors predict long-COVID symptoms. 9/22/22. Son K. Eur Respir J.
Circulating anti-nuclear autoantibodies in COVID-19 survivors predict long-COVID symptoms. 9/22/22. Son K. Eur Respir J.
This longitudinal observational multi-institutional Canadian study of 106 COVID-19 patients from 8/2020 to 9/2021 examined the relationship between long COVID symptoms and antinuclear/extractable-nuclear antibodies (ANA/ENA). Patients, whose acute COVID symptoms varied, were evaluated at 3, 6, and 12 months with ANA/ENA and cytokine assays. They self-reported fatigue, coughing and dyspnea. Controls were age- and sex-matched, nonimmunized, COVID-negative volunteers (22), and non-COVID-19 positive patients with respiratory symptoms (34). At 3 months, ANA levels were increased in COVID patients compared to controls, but decreased over the year. Higher levels of tumor necrosis factor alpha, D-dimer and IL-1β predicted elevated ANA at 12 months. Tracking the increase of antibodies associated with autoimmune disease over time demonstrated potential de novo autoantibody synthesis. The authors conclude that persistently positive ANAs at 12 months post-COVID-19 are associated with continuing symptoms and inflammation in a subset of COVID survivors.
SAB Comment: The authors present elegant immunological evidence of an autoimmune factor in the development of long COVID. However, this small study has much potential for bias. Only 57 of 106 patients were available at 12 months. Also, in a study which concluded severity of disease was important, only 1 of 34 respiratory symptom control patients was hospitalized compared to 80 of 106 COVID-19 patients.- Respiratory Support in the Time of COVID-19. 9/27/22. Nichol AD. JAMA.
This editorial reviews 6 prior randomized studies of non-invasive respiratory support for patients with COVID-19 pneumonitis along with the SOHO-COVID Randomized Clinical Trial, published in the same issue of JAMA. After discussing each study, the authors conclude that “the available evidence suggests that the initial choice of supplemental oxygen therapy for patients with COVID-19-related acute hypoxemic respiratory failure does not influence mortality. This will provide some reassurance for clinicians in times of reduced availability of certain noninvasive respiratory support strategies during surges in COVID-19 hospitalizations.” They found that the rates of intubation using high-flow nasal oxygen vs conventional oxygen therapy did not consistently favor one modality or the other in this group of studies. - COVID-19 mRNA Vaccine Safety Among Children Aged 6 Months-5 Years – United States, June 18, 2022-August 21, 2022. 9/1/22. Hause AM. MMWR Morb Mortal Wkly Rep.
In the time period noted above, 1,040,230 children received Pfizer-BioNTech or Moderna vaccines. Approximately 23,266 children were enrolled in v-safe, the CDC voluntary cell phone-based monitoring system. The most frequent systemic reactions reported to v-safe after receipt of vaccines were irritability or crying in approximately one half of children aged 6 months–2 years. In children aged 3 years or older, systemic reactions after vaccination were less frequently reported; injection site pain was the most frequently reported reaction among these older children. The VAERS reporting system received a total of 1,017 reports of adverse events after Pfizer-BioNTech or Moderna vaccination, 98.1% of which were classified as nonserious. No reports of myocarditis after vaccination were reported. Health care providers and parents of young children should be aware that local and systemic reactions are expected after vaccination with Pfizer-BioNTech or Moderna vaccine and that serious adverse events are rare. - Below you will find a list of additional articles and resources selected for the IARS COVID-19 Resource website, all of which include a summary with major takeaways and are searchable by topic on the website:
Newsletter Issue 148, September 28, 2022:
 Persistent circulating SARS-CoV-2 spike is associated with post-acute COVID-19 sequelae. 9/2/22. Swank Z. Clin Infect Dis.
Persistent circulating SARS-CoV-2 spike is associated with post-acute COVID-19 sequelae. 9/2/22. Swank Z. Clin Infect Dis.
This retrospective pilot study measured 3 SARS-CoV-2 antigens (using a Simoa assay) in blood samples from 63 individuals diagnosed with COVID-19, of whom 37 had postacute sequelae of COVID-19 (PASC) identified. SARS-CoV-2 spike antigen was detected several months postinfection in 60% of PASC and in none with COVID-19 only. Longitudinal blood samples were available in 12 PASC individuals and in 6 with COVID-19 only. Serial blood samples in 12 PASC individuals showed sustained or fluctuating spike antigen levels, whereas in samples of 6 with COVID-19 only, high antigen levels immediately after diagnosis dropped rapidly. The authors indicate that more study is needed and propose these results support the hypothesis that a reservoir of active virus persists within the body in individuals with PASC. Ten cytokines were also measured and were not different among the groups. The discussion further analyzes the validity of the findings.- Nirmatrelvir-Ritonavir and Viral Load Rebound in Covid-19. 9/7/22. Anderson AS. N Engl J Med.
Using data from the EPIC-HR3 trial which compared nirmatrelvir-ritonavir (Paxlovid) to placebo in unvaccinated patients, the authors found that viral load rebound following nirmatrelvir-ritonavir is not retrospectively associated with low nirmatrelvir exposure, recurrence of moderate to severe symptoms or development of resistance to nirmatrelvir. During the recruitment period from June to December 2021 which coincided with delta variant predominance, viral load rebound occurred in 23 of 990 patients (2.3%) in the nirmatrelvir– ritonavir group and in 17 of 980 (1.7%) in the placebo group. These results – published in a research letter suggest that viral load rebound may be a natural feature of a small percentage of SARS CoV-2 infections. However, viral load as determined by PCR assay, may not explain the frequently noted clinical syndrome. - A Bivalent Omicron-Containing Booster Vaccine against Covid-19. 9/16/22. Chalkias S. N Engl J Med.
This industry-sponsored study presents the interim results of Moderna’s phase 2-3 trial of their bivalent mRNA vaccine. Participants (n = 890) who had received a primary series of two doses and a single booster of Moderna’s original vaccine were divided into two groups; one received another booster of the original vaccine, and the other received the new bivalent mRNA vaccine which includes 25mcg of the original, and 25 mcg of an mRNA vaccine developed against Omicron BA-1. At 29 days, the bivalent Omicron-containing vaccine elicited neutralizing antibody responses against Omicron (including BA.4/5 subvariants) and previous variants that were superior to those of the original vaccine, without safety concerns.
SAB Comment: These interim laboratory immune results are encouraging. In the near future we look forward to the important vaccine effectiveness and longevity results. - Below you will find a list of additional articles and resources selected for the IARS COVID-19 Resource website, all of which include a summary with major takeaways and are searchable by topic on the website:
Newsletter Issue 147, September 19, 2022:
 Comparison of Severe Maternal Morbidities Associated With Delivery During Periods of Circulation of Specific SARS-CoV-2 Variants. 8/12/22. Mupanomunda M. JAMA Netw Open.
Comparison of Severe Maternal Morbidities Associated With Delivery During Periods of Circulation of Specific SARS-CoV-2 Variants. 8/12/22. Mupanomunda M. JAMA Netw Open.
Does the incidence of severe maternal morbidity (SMM) vary according to the SARS-CoV-2 variant? In a retrospective study involving 32 US hospitals from March 2020 to January 2022, the authors evaluated SMM in 3,129 mothers with positive RT-PCR results at the delivery hospitalization compared with a propensity-matched cohort of 12,504 mothers who tested negative. SARS-CoV-2 infection at the time of delivery was significantly associated with SMM in the wild-type, Alpha and Delta periods; the magnitude of the associations was most pronounced during the time period when the Delta variant was predominant (OR 7.69). Respiratory (but not nonrespiratory) SMM were significantly higher during the Omicron period. The authors stress the importance of promoting vaccination acceptance and combating misinformation together with other prevention strategies in pregnant individuals.
SAB Comment: As COVID-19 variants, infection patterns, and vaccines evolve, teasing out risks and outcomes has become more intricate. This study focuses on SARS-CoV-2 infection at the time of delivery and provides important information for this particular cohort. An associated commentary helps put the study in perspective and emphasizes the importance of including pregnant patients in the early clinical trials of vaccine safety and efficacy.- Management and outcomes of pregnant women admitted to intensive care unit for severe pneumonia related to SARS-CoV-2 infection: the multicenter and international COVIDPREG study. 8/19/22. Péju E. Intensive Care Med.
The object of this retrospective multicenter international study from France was to assess ventilatory management, as well as obstetric management and neonatal outcomes, of all pregnant women (187) admitted to 32 ICUs with SARS-CoV-2 pneumonia from March 2020 through December 2021. Outcomes were compiled according to trimester of pregnancy, comorbidities, degree of CT scan abnormalities and mode of ventilatory support, among others. Prone positioning was used in 26% of all patients and 56% of intubated patients, but to a lesser degree before delivery. In the 27 intubated, ventilated patients for whom complete data were available, delivery of the neonate significantly improved oxygenation and decreased driving pressure, with a trend toward increased compliance and decreased plateau pressure. Venovenous ECMO was used in 21% of intubated patients. Maternal mortality was 2% and neonatal mortality 4%. Preterm birth occurred in 74% and ICU admission in 54% of neonates of intubated patients. - Below you will find a list of additional articles and resources selected for the IARS COVID-19 Resource website, all of which include a summary with major takeaways and are searchable by topic on the website:
Newsletter Issue 146, September 12, 2022:
 Clinical and Genetic Risk Factors for Acute Incident Venous Thromboembolism in Ambulatory Patients With COVID-19. 8/18/22. Xie J. JAMA Intern Med.
Clinical and Genetic Risk Factors for Acute Incident Venous Thromboembolism in Ambulatory Patients With COVID-19. 8/18/22. Xie J. JAMA Intern Med.
The authors examined data from the UK Biobank for ambulatory patients from March 1, 2020 to September 3, 2021. In this retrospective cohort study of 18,818 outpatients with COVID-19 and 93,179 propensity score-matched noninfected participants, a higher venous thromboembolism (VTE) incidence was observed in the former (hazard ratio, 21.42 (!)); however, this risk was considerably attenuated among the fully vaccinated, after breakthrough infection (hazard ratio, 5.95). Older age, male sex, obesity, no vaccination or partial vaccination, and inherited thrombophilia were independent risk factors for COVID-19–associated VTE. These data help identify high-risk patients for VTE in the ambulatory setting. However, these data predate the Omicron variant.- Neurological and psychiatric risk trajectories after SARS-CoV-2 infection: an analysis of 2-year retrospective cohort studies including 1 284 437 patients. 8/20/22. Taquet M. Lancet Psychiatry.
This retrospective analysis tracks 14 post-COVID neurologic and psychiatric complications in 3 different age groups and the effect of SARS-CoV-2 variants on those complications between January 2021 and April 2022. Using TriNet X, a US based clinical research collaboration platform deployed by a federated network of 62 health care organizations and tracking 89 million anonymized electronic medical record patient data globally, enabled comparison with a propensity matched control group with other non-COVID related respiratory infections. Results indicate that trajectories for mood and anxiety disorders trend lower overall but cognitive defects, dementia, psychotic disorders, and epilepsy remain elevated 2 years after SARS CoV-2 infection among adults, while children are less susceptible overall except for epilepsy. During the Omicron phase of the pandemic, despite less severe disease and lower mortality, neurologic and psychiatric complications continued unabated and pose a considerable future challenge to health care systems.
In an editorial, the use of comparison groups is highlighted as adding valuable perspective to the multifaceted post-Covid symptom complex and prospective studies to validate the results are recommended. - Below you will find a list of additional articles and resources selected for the IARS COVID-19 Resource website, all of which include a summary with major takeaways and are searchable by topic on the website:
- Anticoagulation and Antiplatelet Therapy for Prevention of Venous and Arterial Thrombotic Events in Critically Ill Patients with COVID-19: COVID-PACT. 8/29/22. Bohula EA. Circulation.
- Effect of therapeutic versus prophylactic anticoagulation therapy on clinical outcomes in COVID-19 patients: a systematic review with an updated meta-analysis. 8/23/22. Duo H. Thromb J.
- Prognostic value of von Willebrand factor and ADAMTS13 in patients with COVID-19: A systematic review and meta-analysis. 8/26/22. Xu X. Thromb Res.
- The Association of Baseline Plasma SARS-CoV-2 Nucleocapsid Antigen Level and Outcomes in Patients Hospitalized With COVID-19. 8/29/22. ACTIV-3/TICO Study Group*. Ann Intern Med.
Newsletter Issue 145, September 7, 2022:
Take advantage of COVID-19 Journal CME from newly published peer-reviewed articles from respected journals. The IARS COVID-19 Scientific Advisory Board (SAB) selects articles of the greatest clinical and scientific relevance to anesthesiologists, intensivists, related specialists and investigators. View the Journal CME First-Time User Guide for instructions.
 COVID-19 and Acute Neurologic Complications in Children. 8/11/22. Antoon JW. Pediatrics.
COVID-19 and Acute Neurologic Complications in Children. 8/11/22. Antoon JW. Pediatrics.
This study is based upon data provided to the Pediatric Health Information System database from 52 pediatric academic centers during a 2-year period beginning March 2020. It focused on prevalence, risk factors and outcomes associated with neurological complications in hospitalized children. Among 15,137 children aged 2 months to 18 years, the overall incidence of acute neurological complication was 7%. Febrile seizures, nonfebrile seizures, and encephalopathy combined accounted for 85% of neurologic complications. Identified risk factors included pre-existing chronic neurologic conditions, older age, race, and ethnicity. Lower odds occurred during the period of Delta variant dominance, and in patients treated with remdesivir and dexamethasone. Neurologic complications were associated with increased mortality (1.8% vs. 0.6%, p<0.001), ICU admission, longer hospitalization, and higher cost of care. These results are comparable to children hospitalized with influenza and other viral illnesses and emphasize the importance of COVID-19 immunization, especially in a high-risk population. The Risk of Postoperative Complications After Major Elective Surgery in Active or Resolved COVID-19 in the United States. 2/22/22. Deng JZ. Annals of Surgery.
The Risk of Postoperative Complications After Major Elective Surgery in Active or Resolved COVID-19 in the United States. 2/22/22. Deng JZ. Annals of Surgery.
Using data from the COVID-19 Research Database, investigators studied the incidence of complications following 18 common major elective surgeries in unvaccinated patients who previously had PCR-confirmed SARS-CoV-2 infection (98.7% mild-moderate). Compared with 2,621 controls who underwent equivalent surgeries at least 30 days before a COVID diagnosis, those who had surgery within 4 weeks following a COVID diagnosis (n = 780) had substantially elevated adjusted odds ratio (aOR) for pneumonia (aOR 6.5), respiratory failure (aOR 3.4), pulmonary embolus (aOR 2.4) and sepsis (aOR 3.7). For those having surgery between 4 and 8 weeks after COVID (n = 445), only the aOR for pneumonia was higher at 2.4. For those having surgery more than 8 weeks following COVID (n=1633), no increased risk of complications was observed. Following a COVID diagnosis authors recommend waiting 8 weeks prior to major elective surgery, with due consideration for malignancies. Covid-19 Vaccination and the Timing of Surgery Following Covid-19 Infection. 7/15/22. Le S. Annals of Surgery.
Covid-19 Vaccination and the Timing of Surgery Following Covid-19 Infection. 7/15/22. Le S. Annals of Surgery.
Kaiser Permanente investigators reviewed 228,913 adult elective inpatient and outpatient surgeries, pre-pandemic and after vaccination availability, to assess whether vaccination status or type of anesthesia affected postoperative complication rates following SARS-CoV-2 infection. Postoperative emergency room visits and unscheduled hospitalizations were increased only for patients not fully vaccinated at the time of surgery, and if it occurred <30 days following a COVID diagnosis (n=373, adjusted HR 1.55). The increased risk was fully accounted for in those who had general anesthesia (adjusted HR 1.84). Risks were not elevated when surgery occurred more than 4 weeks following a COVID diagnosis. Authors noted “Current guidelines recommend deferring elective surgery for at least 7 weeks after Covid-19 diagnosis among patients who are asymptomatic at the time of surgery.” They recommend further study and liberalizing guidelines for those fully vaccinated or for whom general anesthesia can be avoided.
SAB Comment: This article prompted SAB review of a key cited reference that analyzed data from unvaccinated patients undergoing major elective surgery earlier in the pandemic. It contributed to guidelines suggesting a 7-8 week preoperative delay following a positive PCR. SARS-CoV-2 continues to evolve with multiple variants, the specter of Long COVID-19, and the concomitant evolution of vaccines and therapeutics. Consequently, decisions regarding timing of elective surgery following a COVID diagnosis are best individualized to each patient. We anticipate ongoing studies regarding the important question of how to assess and minimize perioperative risks following COVID. Maternal Vaccination and Risk of Hospitalization for Covid-19 among Infants. 6/22/22. Halasa NB. N Engl J Med.
Maternal Vaccination and Risk of Hospitalization for Covid-19 among Infants. 6/22/22. Halasa NB. N Engl J Med.
This multicenter, case-control, test-negative study assessed the effectiveness of maternal 2-dose mRNA COVID-19 vaccination during pregnancy against hospitalization for COVID-19 disease among infants younger than 6 months. Protection was 52% overall (80% during the Delta period, and 38% during the Omicron period). Of 537 case infants hospitalized for COVID-19, only 16% were born to mothers fully vaccinated during pregnancy vs. 29% of 512 hospitalized controls. Among case infants, 21% were admitted to ICU; 12% received mechanical ventilation or vasoactive infusions. Two infants died from COVID-19 and 2 received ECMO treatment; none of these 4 infants’ mothers had been vaccinated during pregnancy. Effectiveness was greater if vaccination occurred after 20 weeks of gestational age than earlier in pregnancy (69% vs. 38%). However, due to well-documented risks of COVID-19 during pregnancy, this did not lead to a recommendation to delay vaccination. The results are discussed in a well-written accompanying editorial. Extended prone positioning duration for COVID-19-related ARDS: benefits and detriments. 7/8/22. Walter T. Crit Care.
Extended prone positioning duration for COVID-19-related ARDS: benefits and detriments. 7/8/22. Walter T. Crit Care.
This retrospective observational study in France from March 2020-April 2021 evaluated 81 COVID-19 patients, using a strategy, originally implemented for organizational and human resources purposes. It was based on extending the duration of proning sessions (PP) up to 48 hours in patients ventilated for COVID-19-related ARDS. The primary objective, the occurrence of pressure injury, was observed in 26% of patients and was equivalent to patients who had PP sessions of shorter duration for non-COVID-19-related ARDS. The presence of skin injury, the most common complication of PP, correlated with cumulative duration of PP sessions and length of ICU stay, not the duration of each session. Extended PP significantly reduced staff viral exposure and workload, allowing for most position changes during the daytime. Longer proning sessions were also associated with continued improvements in ventilatory parameters over the extended time. Association between AZD7442 (tixagevimab-cilgavimab) Administration and SARS-CoV-2 Infection, Hospitalization and Mortality. 7/29/22. Kertes J. Clin Infect Dis.
Association between AZD7442 (tixagevimab-cilgavimab) Administration and SARS-CoV-2 Infection, Hospitalization and Mortality. 7/29/22. Kertes J. Clin Infect Dis.
This retrospective, observational, “real world” study demonstrates lower rates of infection (3.5% vs. 7.2%), hospitalization (0.1 vs. 0.6%) and mortality (0 vs. 0.9%) when AZD7442 is administered pre-infection to a heterogenous group of highly immunosuppressed patients and compared to a similar group who chose not to receive the drug combination. The study coincided with an Omicron-dominated wave of COVID-19 in Israel (Dec. 2021- April 2022) and was designed to determine whether AZD7442, a human SARS-CoV-2 neutralizing monoclonal antibody combination, with the US trade name, Evusheld, was effective against the Omicron variant using a large Israeli healthcare system’s database. Among the immune suppressed patients contacted, 825 patients chose to participate and received AZD7442, while 4,299 declined and served as controls. Study results complement several ongoing and completed randomized controlled trials (PROVENT, TACKLE) and suggest the use of AZD7442 with its 90-day half-life for pre-exposure prophylaxis in individuals where immune-suppression is a concern. Good practice statements for antithrombotic therapy in the management of COVID-19: Guidance from the SSC of the ISTH. 7/29/22. J Thromb Haemost. Clin Infect Dis.
Good practice statements for antithrombotic therapy in the management of COVID-19: Guidance from the SSC of the ISTH. 7/29/22. J Thromb Haemost. Clin Infect Dis.
This article is a set of best practice statements for deep vein thrombosis prophylaxis and treatment with COVID-19 from the International Society of Thrombosis and Hemostasis. It is not based upon a systematic review but expert opinion. While much of this is similar to prior guidelines, the special considerations section covering pregnancy, pediatrics, chronic kidney disease, obesity and heparin-induced thrombocytopenia without thrombosis are of value.
Newsletter Issue 144, August 29, 2022:
 COVID-19 and Acute Neurologic Complications in Children. 8/11/22. Antoon JW. Pediatrics.
COVID-19 and Acute Neurologic Complications in Children. 8/11/22. Antoon JW. Pediatrics.
This study is based upon data provided to the Pediatric Health Information System database from 52 pediatric academic centers during a 2-year period beginning March 2020. It focused on prevalence, risk factors and outcomes associated with neurological complications in hospitalized children. Among 15,137 children aged 2 months to 18 years, the overall incidence of acute neurological complication was 7%. Febrile seizures, nonfebrile seizures, and encephalopathy combined accounted for 85% of neurologic complications. Identified risk factors included pre-existing chronic neurologic conditions, older age, race, and ethnicity. Lower odds occurred during the period of Delta variant dominance, and in patients treated with remdesivir and dexamethasone. Neurologic complications were associated with increased mortality (1.8% vs. 0.6%, p<0.001), ICU admission, longer hospitalization, and higher cost of care. These results are comparable to children hospitalized with influenza and other viral illnesses and emphasize the importance of COVID-19 immunization, especially in a high-risk population.- Oral Nirmatrelvir and Ritonavir in Non-hospitalized Vaccinated Patients with Covid-19. 8/20/22. Ganatra S. Clin Infect Dis.
Though Paxlovid (nirmatrelvir plus ritonavir, NMV-r) reduces progression to severe disease in high-risk, nonhospitalized unvaccinated patients, benefits among vaccinated patients are unclear. This retrospective cohort study between December 2021 and April 2022 evaluated vaccinated adults (over 18 years), who subsequently developed COVID-19, comparing 1,130 who took NMV-r within five days of diagnosis with an equal number who did not. All-cause emergency room visits, hospitalization, or death within 30 days occurred in 89 patients (7.87%) in the NMV-r cohort, compared to 163 patients (14.4%) in the non-NMV-r cohort (p<0.005), a 45% relative risk reduction. Complications and overall resource utilization (e.g., diagnostic tests) were also decreased. - Nirmatrelvir Use and Severe Covid-19 Outcomes during the Omicron Surge. 8/24/22. Arbel R. N Engl J Med.
This observational, retrospective, Israeli study of ambulatory patients, conducted January 9, 2022 – March 31, 2022 during the Omicron period, investigated early use of nirmatrelvir, a protease inhibitor. Of 105,352 eligible patients (over 40 yrs of age and considered “high risk”), 3,902 received the drug. The authors report significantly lower rates of hospitalization and death within 35 days in patients over 65 yrs who received nirmatrelvir (HR 0.27) irrespective of previous immunity status. They also noted a further reduction of hospitalization in patients over 65 who had previous immunity to COVID from vaccination, previous infection or both, and received the drug. No benefit of use was noted in patients less than 65 years of age. - Levels of SARS-CoV-2 antibodies among fully vaccinated individuals with Delta or Omicron variant breakthrough infections. 8/1/22. Breinholt Stærke N. Nature Communications.
Using the Danish National Microbiology database, this observational cohort study examined whether levels of anti-spike IgG influenced rates of Delta and Omicron breakthrough infections in a fully vaccinated cohort. Anti-spike IgG levels were measured before vaccinations and at days 21–28, 90, 180 post first-vaccination, and 4 weeks post-booster. Among 6076 participants, Delta and Omicron (BA.2) infections occurred in 127 and 364, respectively. The highest vs. lower quintile of anti-spike IgG led to a 71% lower Delta breakthrough infection rate (p = 0.0002). However, no significant quintile differences were observed for Omicron suggesting that the level of anti-spike IgG has limited impact on Omicron breakthrough risk.
SAB Comment: Prior to Omicron’s emergence, and consistent with the Delta variant data in the above study, increasing levels of anti-spike IgG were associated with reduced risk of breakthrough infections. This observation is not valid with Omicron breakthrough infection and does not address T- and B-cell immune function post-vaccination. - Below you will find a list of additional articles and resources selected for the IARS COVID-19 Resource website, all of which include a summary with major takeaways and are searchable by topic on the website:
Newsletter Issue 143, August 22, 2022:
 Good practice statements for antithrombotic therapy in the management of COVID-19: Guidance from the SSC of the ISTH. 7/29/22. J Thromb Haemost. Clin Infect Dis.
Good practice statements for antithrombotic therapy in the management of COVID-19: Guidance from the SSC of the ISTH. 7/29/22. J Thromb Haemost. Clin Infect Dis.
This article is a set of best practice statements for deep vein thrombosis prophylaxis and treatment with COVID-19 from the International Society of Thrombosis and Hemostasis. It is not based upon a systematic review but expert opinion. While much of this is similar to prior guidelines, the special considerations section covering pregnancy, pediatrics, chronic kidney disease, obesity and heparin-induced thrombocytopenia without thrombosis are of value.- ISTH guidelines for antithrombotic treatment in COVID-19. 7/29/22. Schulman S. J Thromb Haemost.
This article is a systematic review and guidelines for deep vein thrombosis prophylaxis with COVID-19 from the International Society of Thrombosis and Hemostasis. It was performed by a panel of experts. Among noncritically ill patients hospitalized for COVID-19, the panel gave a strong recommendation (a) for use of prophylactic dose of low molecular weight heparin or unfractionated heparin (LMWH/UFH) (COR 1); (b) for select patients in this group, use of therapeutic dose LMWH/UFH in preference to prophylactic dose (COR 1); but (c) against the addition of an antiplatelet agent (COR 3). Nine additional recommendations are made. The review is comprehensive and current, and the recommendations are graded. Figure 2 is useful as it summarizes the findings. - Long COVID: which symptoms can be attributed to SARS-CoV-2 infection? 8/7/22. Brightling CE. Lancet.
This brief comment places into perspective a long COVID study by Ballering et al, and provides a nutshell view of long COVID. The Ballering study found a long COVID incidence of 12.7% in a geographically and ethnically limited study that did not consider mental health. It also introduces a possible core symptom set for diagnosis. The authors consider the methodology a “major advance on previous long COVID prevalence estimates.” Important concepts introduced in the comment include: “clustering of patient-reported outcomes has identified different severity groups of long COVID and identified increased systemic inflammation in people with very severe long COVID,”; “the proportion of newly infected people developing long COVID is reduced in people who have received vaccination before SARS-CoV-2 infection,” and ”might be lower in people infected with the omicron variant than those infected with earlier variants.” “Current evidence supports the view that long COVID is common and can persist for at least 2 years after SARS-CoV-2 infection, although severe debilitating disease is present in a minority.” - Risk of SARS-CoV-2 Acquisition in Health Care Workers According to Cumulative Patient Exposure and Preferred Mask Type. 8/15/22. Dörr T. JAMA Netw Open.
In this study of 2,919 Swiss healthcare workers over a 1-year period beginning September 2020, the incidence of SARS-CoV-2 positivity was correlated with cumulative hours of exposure to patients with COVID-19 and lower with exclusive use of FFP2 respirator masks (similar to N95) vs. surgical/mixed mask use. Positivity rates (by testing and/or antinucleocapsid Ab seroconversion) was 13% for workers with no patient contact, 21% for workers caring for COVID patients using respirator masks exclusively and 35% for workers who used a combination of masks. Other significant factors were positive household contacts (OR 7.8), and vaccination (OR 0.55), but not working in ICU.
SAB Comment: Although this study has weaknesses, including self-reported data, we have seen few others reporting the relative benefits of respirator mask wearing. Given the current availability of respirator masks to consumers along with the high rate of breakthrough infections of the very infectious BA.5 Omicron variant, these data may be useful to all who wish to optimize COVID protection in crowded, indoor, and travel environments in addition to use in healthcare settings. - Effectiveness of mRNA COVID-19 vaccines against Omicron and Delta variants in a matched test-negative case-control study among US veterans. 8/3/22. Young-Xu Y. BMJ Open.
This study used VA electronic health record data from 114,640 veterans who had a SARS-CoV-2 test during November 2021 or January 2022 to calculate estimates of vaccine effectiveness and considered November infections to be due to the Delta variant and January infections to be due to the Omicron variant.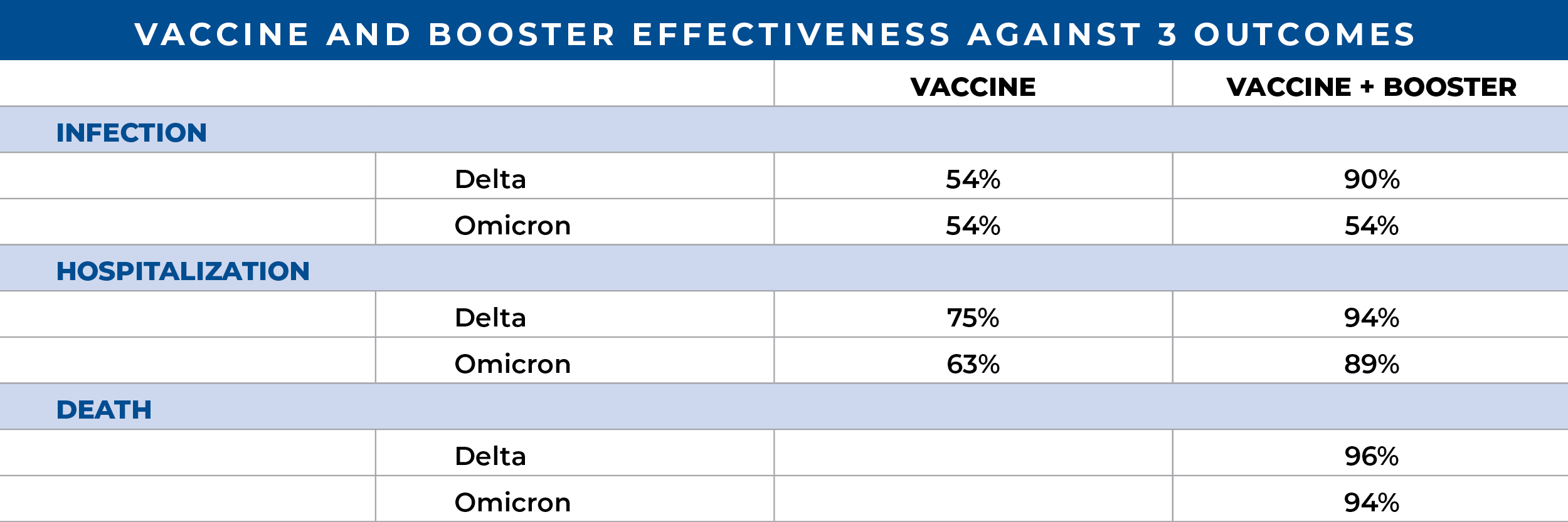
The authors concluded that although the effectiveness of booster vaccination against infection was moderately higher against Delta than against the early Omicron SARS-CoV-2 variant, effectiveness against severe disease and death was similarly high against both variants. - An Antibody from Single Human VH-rearranging Mouse Neutralizes All SARS-CoV-2 Variants Through BA.5 by Inhibiting Membrane Fusion. 8/11/22. Luo S. Science Immunology.
Anti-spike-directed vaccines and therapeutic antibodies are increasingly ineffective. Using a humanized mouse model, laboratory investigators inserted human gene segments that promoted mouse B-cells to produce humanized antibody repertoires using “V(D)J recombination,” like humans build antibodies after antigen exposures. Novel anti-spike antibodies were then produced following ancestral spike protein exposure. One antibody, SP1-77, neutralized Alpha, Beta, Gamma, Delta, and all Omicron strains. Though binding to the RBD, SP1-77 does not block ACE2 receptor binding. Shown using groundbreaking imaging, SP1-77 works through a novel downstream mechanism, preventing fusion of virus outer membranes with target cell membranes inhibiting a thus far unmuted RBD site.
SAB Comment: The SP1-77 antibody anti-fusion mechanism may provide insights to future vaccine strategies. In this preclinical study, the humanized “VH1-2/Vκ1-33-rearranging mouse model” yielded diverse human heavy and light chain rearrangements and resultant novel immunoglobulins. Application of these experimental techniques can lead to therapeutic antibody repertoire discoveries against future SARS-Co-V variants or any pathogen (e.g., HIV-1, influenza). - Below you will find a list of additional articles and resources selected for the IARS COVID-19 Resource website, all of which include a summary with major takeaways and are searchable by topic on the website:
- Association of Blood Viscosity With Mortality Among Patients Hospitalized With COVID-19. 7/21/22. Choi D. J Am Coll Cardiol.
- Respiratory indications for ECMO: focus on COVID-19. 8/9/22. Supady A. Intensive Care Med.
- Baricitinib in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial and updated meta-analysis. 7/31/22. RECOVERY Collaborative Group. Lancet.
- C reactive protein utilisation, a biomarker for early COVID-19 treatment, improves lenzilumab efficacy: results from the randomised phase 3 ‘LIVE-AIR’ trial. 7/6/22. BMJ. Temesgen Z.
Newsletter Issue 142, August 15, 2022:
 Association between AZD7442 (tixagevimab-cilgavimab) Administration and SARS-CoV-2 Infection, Hospitalization and Mortality. 7/29/22. Kertes J. Clin Infect Dis.
Association between AZD7442 (tixagevimab-cilgavimab) Administration and SARS-CoV-2 Infection, Hospitalization and Mortality. 7/29/22. Kertes J. Clin Infect Dis.
This retrospective, observational, “real world” study demonstrates lower rates of infection (3.5% vs. 7.2%), hospitalization (0.1 vs. 0.6%) and mortality (0 vs. 0.9%) when AZD7442 is administered pre-infection to a heterogenous group of highly immunosuppressed patients and compared to a similar group who chose not to receive the drug combination. The study coincided with an Omicron-dominated wave of COVID-19 in Israel (Dec. 2021- April 2022) and was designed to determine whether AZD7442, a human SARS-CoV-2 neutralizing monoclonal antibody combination, with the US trade name, Evusheld, was effective against the Omicron variant using a large Israeli healthcare system’s database. Among the immune suppressed patients contacted, 825 patients chose to participate and received AZD7442, while 4,299 declined and served as controls. Study results complement several ongoing and completed randomized controlled trials (PROVENT, TACKLE) and suggest the use of AZD7442 with its 90-day half-life for pre-exposure prophylaxis in individuals where immune-suppression is a concern.- Efficacy and safety of intramuscular administration of tixagevimab-cilgavimab for early outpatient treatment of COVID-19 (TACKLE): a phase 3, randomised, double-blind, placebo-controlled trial. 6/10/22. Montgomery H. Lancet Respir Med.
This human SARS-CoV-2-neutralizing monoclonal antibody (NMA) combination, also referred to as AZD7442 or Evusheld, has an extended half-life of 90 days and is subject to an Astra Zeneca-funded, multicenter, international trial which began enrolling unvaccinated patients with seven days or less of mild COVID-19 symptoms and at least one major risk factor in January 2021. By July 2021, 910 patients (average age 46) had received either placebo (n = 454) or an intramuscular injection of 300 mg each of tixagevimab and cilgavimab (n = 456). Progression to severe illness or death from any cause through day 29 occurred in 18 patients receiving the NMA combination, compared to 36 who received placebo, resulting in a relative risk reduction of 50.5%.
In the pre-Omicron era, this NMA combination provided significant protection from severe disease without major side effects. As the trial is ongoing, future results may confirm in-vitro effectiveness to the Omicron variant.
SAB Comment: An associated editorial, titled “Early treatment to prevent progression of SARS-CoV-2 infection,” highlights the evolving role antivirals and monoclonal antibodies like Evusheld may play in the prevention and management of SARS-CoV-2 and its variants, and the protection of vulnerable populations from severe disease. - Association of nirmatrelvir/ritonavir treatment on upper respiratory SARS-CoV-2 RT-PCR negative conversion rates among high-risk patients with COVID-19. 7/23/22. Li H. Clin Infect Dis.
Nirmaltrelvir/ritonavir (Paxlovid) given orally within 5 days of symptom onset to high-risk patients hospitalized with mild to moderate COVID-19, accelerated the RT-PCR conversion rate from positive to negative significantly. Median conversion time for a 258-patient cohort receiving the drug between March and April 2022 was day 10 (7-12 days) while a control cohort of 241 patients converted 7 days later on day 17 (12-21 days). Secondary outcomes included lingering PCR positivity on day 15 among untreated patients, resulting in a hazard ratio of 4.33 in favor of patients receiving the drug combination. Delayed negative conversion were also observed in patients with lower cycle threshold (Ct) during RT-PCR testing, suggesting the presence of greater viral loads. Although neither infectiousness nor diminishing viral loads were assessed directly, the accelerated RT-PCR conversion suggests reduced risk of viral shedding and disease transmission in patients receiving the antiviral. - Efficacy of Antibodies and Antiviral Drugs against Omicron BA.2.12.1, BA.4, and BA.5 Subvariants. 7/20/22. N Engl J Med.
The efficacy of monoclonal antibodies against the circulating BA.2.12.1, BA.4 and BA.5 is unknown. Live virus neutralization laboratory testing showed the combination of casirivimab and imdevimab (REGEN-COV) inhibited all variants; however, neutralization was reduced vs. imdevimab alone. Casirivmab was inactive. The combination of tixagevimab and cilgavimab (Evusheld) was active vs all three variants as was cilgavimab. Tixagevimab only neutralized BA.2.12.1. Sotrovimab lost all inhibitory capability. Bebtelovimab neutralized all three and was the only monoclonal active at concentrations similar to the ancestral strain. Remdesivir, molnupiravir and nirmatrelvir (active component of Paxlovid) all neutralized variants at concentrations similar to ancestral stains.
SAB Comment: To date, there has been a lack of in vitro or clinical data to help decision making in selection of monoclonals and antiviral drugs to treat current circulating variants. These in vitro data may help a treating physician formulate a rationale for such decisions.
Newsletter Issue 141, August 8, 2022:
 Covid-19 Vaccination and the Timing of Surgery Following Covid-19 Infection. 7/15/22. Le S. Annals of Surgery.
Covid-19 Vaccination and the Timing of Surgery Following Covid-19 Infection. 7/15/22. Le S. Annals of Surgery.
Kaiser Permanente investigators reviewed 228,913 adult elective inpatient and outpatient surgeries, pre-pandemic and after vaccination availability, to assess whether vaccination status or type of anesthesia affected postoperative complication rates following SARS-CoV-2 infection. Postoperative emergency room visits and unscheduled hospitalizations were increased only for patients not fully vaccinated at the time of surgery, and if it occurred <30 days following a COVID diagnosis (n=373, adjusted HR 1.55). The increased risk was fully accounted for in those who had general anesthesia (adjusted HR 1.84). Risks were not elevated when surgery occurred more than 4 weeks following a COVID diagnosis. Authors noted “Current guidelines recommend deferring elective surgery for at least 7 weeks after Covid-19 diagnosis among patients who are asymptomatic at the time of surgery.” They recommend further study and liberalizing guidelines for those fully vaccinated or for whom general anesthesia can be avoided.
SAB Comment: This article prompted SAB review of a key cited reference that analyzed data from unvaccinated patients undergoing major elective surgery earlier in the pandemic. It contributed to guidelines suggesting a 7-8 week preoperative delay following a positive PCR. SARS-CoV-2 continues to evolve with multiple variants, the specter of Long COVID-19, and the concomitant evolution of vaccines and therapeutics. Consequently, decisions regarding timing of elective surgery following a COVID diagnosis are best individualized to each patient. We anticipate ongoing studies regarding the important question of how to assess and minimize perioperative risks following COVID. The Risk of Postoperative Complications After Major Elective Surgery in Active or Resolved COVID-19 in the United States. 2/22/22. Deng JZ. Annals of Surgery.
The Risk of Postoperative Complications After Major Elective Surgery in Active or Resolved COVID-19 in the United States. 2/22/22. Deng JZ. Annals of Surgery.
Using data from the COVID-19 Research Database, investigators studied the incidence of complications following 18 common major elective surgeries in unvaccinated patients who previously had PCR-confirmed SARS-CoV-2 infection (98.7% mild-moderate). Compared with 2,621 controls who underwent equivalent surgeries at least 30 days before a COVID diagnosis, those who had surgery within 4 weeks following a COVID diagnosis (n = 780) had substantially elevated adjusted odds ratio (aOR) for pneumonia (aOR 6.5), respiratory failure (aOR 3.4), pulmonary embolus (aOR 2.4) and sepsis (aOR 3.7). For those having surgery between 4 and 8 weeks after COVID (n = 445), only the aOR for pneumonia was higher at 2.4. For those having surgery more than 8 weeks following COVID (n=1633), no increased risk of complications was observed. Following a COVID diagnosis authors recommend waiting 8 weeks prior to major elective surgery, with due consideration for malignancies.- Dendrimer nanotherapy for severe COVID-19 attenuates inflammation and neurological injury markers and improves outcomes in a phase2a clinical trial. 7/20/22. Gusdon AM. Science Trans Med.
OP-101, a therapeutic nanoparticle with attached “tendrils” (a dendrimer), can place high concentrations of the anti-inflammatory/antioxidant N-acetyl cysteine (NAC) into activated macrophages and microglia. This multicenter, randomized, double-blind, phase 2a pilot study tested single-escalating doses (2mg/kg: n=6, 4mg/kg: n=6, or 8 mg/kg: n=5) vs placebo (n = 7) in severe COVID-19. All received SOC/corticosteroids. OP-101 at 4 mg/kg significantly decreased inflammatory markers; 4 and 8 mg/kg reduced neurological injury markers. Risk for mechanical ventilation/death at 30 and 60 days was 71% for placebo and 18% for pooled OP-101. At 60 days, 3/7 placebo-treated patients and 14/17 OP-101–patients survived without drug-related adverse events.
SAB Comment: Multiple ligands can be attached to injectable dendrimer nanoparticles to allow the novel targeting, treating, and even illuminating of specific inflammatory cells. In this first-in-human trial, macrophages and microglia, suspected culprits in COVID-19, were nanotherapy-targeted and “treated” with high concentration of NAC. The striking, potentially “game-changing” survival results reported here in severe, corticosteroid-treated COVID-19 requires confirmation in much larger multi-institutional studies. Neuropsychiatric exams should be included. - Effectiveness Associated With Vaccination After COVID-19 Recovery in Preventing Reinfection. 7/27/22. Lewis N. JAMA Netw Open.
In this cohort study of more than 95,000 Rhode Island residents from March 2020 to December 2021, including residents and employees of long-term congregate care (LTCC) facilities, completion of the primary vaccination series after recovery from COVID-19 was associated with 49% protection from reinfection among LTCC residents, 47% protection among LTCC employees, and 62% protection in the general population during periods when wild type, Alpha, and Delta strains of SARS-CoV-2 were predominant.
SAB Comment: The finding that in people who have recovered from COVID-19, subsequent completion of the primary vaccination series reduced the risk of reinfection by approximately half is shown here for the earlier variants, but that conclusion may not be extended to the Omicron variants without further study. - Below you will find a list of additional articles and resources selected for the IARS COVID-19 Resource website, all of which include a summary with major takeaways and are searchable by topic on the website:
Newsletter Issue 140, August 3, 2022:
 Extended prone positioning duration for COVID-19-related ARDS: benefits and detriments. 7/8/22. Walter T. Crit Care.
Extended prone positioning duration for COVID-19-related ARDS: benefits and detriments. 7/8/22. Walter T. Crit Care.
This retrospective observational study in France from March 2020-April 2021 evaluated 81 COVID-19 patients, using a strategy, originally implemented for organizational and human resources purposes. It was based on extending the duration of proning sessions (PP) up to 48 hours in patients ventilated for COVID-19-related ARDS. The primary objective, the occurrence of pressure injury, was observed in 26% of patients and was equivalent to patients who had PP sessions of shorter duration for non-COVID-19-related ARDS. The presence of skin injury, the most common complication of PP, correlated with cumulative duration of PP sessions and length of ICU stay, not the duration of each session. Extended PP significantly reduced staff viral exposure and workload, allowing for most position changes during the daytime. Longer proning sessions were also associated with continued improvements in ventilatory parameters over the extended time.- The COVID-19 pandemic and its consequences for chronic pain: a narrative review. 7/18/22. Shanthanna H. Anaesthesia.
This is a narrative review of 3,859 published reports. It succinctly reviews new onset chronic pain after SARS-CoV-2 infection, the influence of the pandemic on patients with pre-existing chronic pain, possible pain mechanisms associated with SARS-CoV-2, and treatments being considered to address post-COVID-19 pain. Both new onset post-COVID-19 pain and ongoing chronic pain increased. Risk factors were social isolation, lack of psychological support, female sex, lower level of education, reduced physical activity and disabled employment status. Tables summarize key findings on COVID-19-associated pain and headache, and the effect of the pandemic on chronic pain patients and the use of steroids for pain interventions. A diagram illustrates possible mechanisms of pain and associated symptoms with COVID-19. Finally, vaccination and the use of steroids and opioids are discussed. - Antibody evasion by SARS-CoV-2 Omicron subvariants BA.2.12.1, BA.4, & BA.5. 7/5/22. Wang Q. Nature.
The authors performed systematic antigenic analyses of Omicron subvariants finding BA.2.12.1 only modestly (1.8-fold) more resistant to sera from vaccinated and boosted individuals than BA.2. However, BA.4/5 is substantially (4.2-fold) more resistant and thus more likely to lead to vaccine breakthrough infections. Among therapeutic antibodies authorized for clinical use, only bebtelovimab retains full potency against both BA.2.12.1 and BA.4/5. Serum neutralization assays used sera from persons who received three shots of mRNA vaccines, others who received mRNA vaccines before or after non-Omicron infection, vaccinated patients with BA.1 or BA.2 breakthrough infection as well as pseudoviruses containing point mutations. The Omicron SARS-CoV-2 lineage continues to evolve, successively yielding subvariants that are both more transmissible and more evasive to antibodies.
SAB Comment: Bebtelovimab (Eli Lilly) has been authorized under EUA since 2/22 for mild-to-moderate COVID-19 in patients older than 12 years and weighing at least 40 kg who are at high risk for progression to severe disease. It is given as a single IV injection, within 7 days of symptom onset. Readers may access current NIH Therapeutic Guidelines, including use of bebtelovimab, on the IARS COVID-19 Published Guidelines and Reviews web page. - Below you will find a list of additional articles and resources selected for the IARS COVID-19 Resource website, all of which include a summary with major takeaways and are searchable by topic on the website:
Newsletter Issue 139, July 18, 2022:
- Viral dynamics of Omicron and Delta SARS-CoV-2 variants with implications for timing of release from isolation: a longitudinal cohort study. 7/23/22. Bouton TC. Clin Infect Dis.
Starting January 2022, CDC guidelines recommended isolation for 5 days from symptom onset, or a positive test if asymptomatic, followed by 5 days of masking. This university campus, longitudinal study (n=92 subjects, mean age 22, 17 Delta, 75 Omicron) examined 10 consecutive days of PCR and antigen tests vs culture positivity. Beyond day 5, 17% remained culture positive (latest day 12) and no difference in time to culture conversion by variant or vaccination status. Most culture-converted by day 6. Conclusion: antigen testing may provide reassurance of lack of infectiousness, though masking days 6-10 is supported by 17% that remained culture positive after day 5.
SAB Comment: In this study, culture positivity was a surrogate for infectiousness, which is a reasonable assumption, but never confirmed. However, it does suggest that in an otherwise “healthy” group in their early twenties (e.g., college students, medical students) a negative antigen test is reassuring irrespective of strain, but mask-wearing remains advisable until day 10 post-symptom onset. This information on the value of antigen tests should not necessarily be extrapolated to other cohorts including older individuals, immunocompromised, and those many months out from primary vaccination or boosters.  Maternal Vaccination and Risk of Hospitalization for Covid-19 among Infants. 6/22/22. Halasa NB. N Engl J Med.
Maternal Vaccination and Risk of Hospitalization for Covid-19 among Infants. 6/22/22. Halasa NB. N Engl J Med.
This multicenter, case-control, test-negative study assessed the effectiveness of maternal 2-dose mRNA COVID-19 vaccination during pregnancy against hospitalization for COVID-19 disease among infants younger than 6 months. Protection was 52% overall (80% during the Delta period, and 38% during the Omicron period). Of 537 case infants hospitalized for COVID-19, only 16% were born to mothers fully vaccinated during pregnancy vs. 29% of 512 hospitalized controls. Among case infants, 21% were admitted to ICU; 12% received mechanical ventilation or vasoactive infusions. Two infants died from COVID-19 and 2 received ECMO treatment; none of these 4 infants’ mothers had been vaccinated during pregnancy. Effectiveness was greater if vaccination occurred after 20 weeks of gestational age than earlier in pregnancy (69% vs. 38%). However, due to well-documented risks of COVID-19 during pregnancy, this did not lead to a recommendation to delay vaccination. The results are discussed in a well-written accompanying editorial.- Below you will find a list of additional articles and resources selected for the IARS COVID-19 Resource website, all of which include a summary with major takeaways and are searchable by topic on the website:
- When to test for COVID-19 using RT-PCR: a systematic review. 6/27/22. Dos Santos PG. Int J Infect Dis.
- Long COVID symptoms in SARS-CoV-2-positive children aged 0-14 years and matched controls in Denmark (LongCOVIDKidsDK): a national, cross-sectional study. 6/25/22. Kikkenborg Berg S. Lancet Child Adolesc Health.
Newsletter Issue 138, July 11, 2022:
- Oral Sabizabulin for High-Risk, Hospitalized Adults with Covid-19: Interim Analysis. 7/6/22. Barnette KG. NEJM Evidence.
Sabizabulin is a novel, oral, microtubule disruptor with antiviral and anti-inflammatory activities. Investigators studied sabizabulin for up to 21 days in a randomized (2:1 vs. placebo), multinational phase 3 trial, in hospitalized patients with moderate to severe COVID-19 at high risk of ARDS and death. After 204 patients were assigned, the trial was stopped for efficacy when a planned interim analysis of 150 patients revealed 60-day mortality (primary end point) was 20.2% (19 of 94) for sabizabulin vs. 45.1% (23 of 51) for placebo, an absolute reduction of 25%, and a 55% relative mortality reduction. Reduced mortality was seen regardless of concomitant treatments such as prednisone and remdesivir, baseline WHO ordinal score, sex, age, comorbidities, or geographic location (US or mostly Brazil). Secondary end points including days of hospitalization, ICU, and mechanical ventilation all strongly and significantly favored sabizabulin, as did serious adverse events.
SAB Comment: This study, conducted from May 18, 2021 to January 31, 2022, and sponsored by sabizabulin’s developer, Veru Inc., showed a striking 55% relative mortality reduction vs. placebo. Colchicine, also a microtubule inhibitor, failed in clinical trials. Sabizabulin is touted to have unique physiochemical properties. Of note, the placebo mortality rate is higher than other recent severe COVID studies. The small study size begs for larger independent studies to confirm potentially exciting, practice-changing results. Veru has applied to the FDA for an Emergency Use Authorization. - Hospitalization and Emergency Department Encounters for COVID-19 After Paxlovid Treatment – California, December 2021-May 2022. 6/23/22. Malden DE. MMWR Morb Mortal Wkly Rep.
To assess hospital admissions and emergency care visits after a course of Paxlovid using the Kaiser Permanente Health Care System’s database, the authors found that among 5,200 patients with mild to moderate disease, advanced age or concomitant medical conditions, only 6 patients (0.11%) treated with Paxlovid were identified with COVID-19-related hospitalization and 39 (0.74 %) had ED encounters 5-15 days after treatment was dispensed.
The likely mechanisms causing the recurrence of COVID-19 symptoms after early treatment with Paxlovid are transient suppression of viral replication before natural immunity is sufficient to complete viral clearance. This could not be proven since viral sequencing was not done and recovery from the initial infection was not assessed. Less likely and also not proven in this study are viral reinfection or emergence of treatment-resistant mutations. - Below you will find a list of additional articles and resources selected for the IARS COVID-19 Resource website, all of which include a summary with major takeaways and are searchable by topic:
- Benefits of plasma exchange on mortality in patients with COVID-19: a systematic review and meta-analysis. 6/16/22. Qin J. Int J Infect Dis.
- American Society of Hematology living guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID-19: March 2022 update on the use of anticoagulation in critically ill patients. 6/24/22. Cuker A. Blood Adv.
Newsletter Issue 137, July 6, 2022:
- N95 respirators: quantitative fit test pass rates and usability and comfort assessment by health care workers. 5/29/22. Ng I. Medical Journal of Australia.
Healthcare workers (n = 2,161) at the Royal Melbourne Hospital underwent quantitative fit testing of N95 respirators per Australian Infection Control Expert Group protocol, each with at least three of four types: semi-rigid cup, flat-fold cup, duckbill, and three-panel flat-fold. Images are available here. The overall fit test pass rates were 65% for the semi-rigid cup respirators, 32% for the flat-fold respirator, 59% for the duckbill respirators, and 96% for the three-panel flat-fold respirator. Three hundred seventy-eight participants completed a detailed comfort and usability survey. Overall comfort and assessment ratings differed significantly by type. Median overall comfort and overall assessment values were highest for the three-panel flat-fold model and lowest for the semi-rigid cup model. These results may inform institutional procurement decision-makers as well as individuals who may not have access to quantitative testing to enhance respiratory protection. - Evolving outcomes of extracorporeal membrane oxygenation during the first 2 years of the COVID-19 pandemic: a systematic review and meta-analysis. 5/23/22. Ling RR. Crit Care.
This article reviews the extracorporeal membrane oxygenation (ECMO) experience worldwide using a meta-analysis and systematic review. Of 4,522 citations, they included 52 studies comprising 18,211 patients. The pooled mortality rate among patients with COVID-19 requiring ECMO was 48.8%. Mortality was higher among studies which enrolled patients later in the pandemic as opposed to earlier (1st half 2020: 41.2%, 2nd half 2020: 46.4%, 1st half 2021: 62.0%, 2nd half 2021: 46.5%, p value = 0.0014). Predictors of increased mortality included age, an increased proportion of patients receiving corticosteroids, and a shorter duration of ECMO run. These data help characterize changes in COVID-19 with respect to outcome using ECMO. - Short-term, relative effectiveness of four doses versus three doses of BNT162b2 vaccine in people aged 60 years and older in Israel: retrospective, test negative, case-control study. 5/24/22. Gazit S. BMJ.
Patients older than 60 years from a single Israeli healthcare provider were studied during Omicron dominance to determine the relative effectiveness of 4 vs. 3 Pfizer vaccine doses against a +PCR, and against COVID-related hospitalization or death. Approximately twenty-eight thousand people received the 4th dose and were compared to ~78,000 who were eligible but had not. A 4th dose provided peak relative protection from infection of 65% at week 3; however, by week 10 it was only 22% greater than 3 doses. Relative protection from severe disease or death, both occurring in <1% of subjects, was more durable in the 4th dose group, remaining >72% greater throughout the 10-week study period. - ASA and APSF Statement on Perioperative Testing for the COVID-19 Virus. 6/16/22. ASA & APSF.
SAB Comment: Readers are encouraged to review the full publication. A few key points, including the potential use of community transmission metrics to guide the use of universal testing, are summarized below:
SCREENING: All patients should be screened for COVID-19 symptoms and for close contact to someone diagnosed with COVID-19 in the past 10 days.
TESTING: The use and limitations of PCR for SARS-CoV-2 testing are reviewed. If a patient tests positive, elective surgical procedures should be delayed. Antibody testing is not recommended for perioperative screening.
COMMUNITY TRANSMISSION METRICS: Two metrics, based on cases/100K in the last 7 days and % test positivity in the last 7 days, are available by community on the CDC website. When levels are graded substantial to high, universal use of PCR testing is recommended. If community transmission is low to moderate, the patient is asymptomatic, up-to-date in vaccination and having a lower-risk procedure, facilities could consider a more permissive approach to perioperative testing.
A number of specific situations are reviewed, including cases of immunosuppressed or other patients likely to carry transmissible virus for longer than 10 days after infection. - Below you will find a list of additional articles and resources selected for the IARS COVID-19 Resource website, all of which include a summary with major takeaways and are searchable by topic:
- Efficacy and safety of intramuscular administration of tixagevimab-cilgavimab for early outpatient treatment of COVID-19 (TACKLE): a phase 3, randomised, double-blind, placebo-controlled trial. 6/10/22. Montgomery H. Lancet Respir Med.
- Impact of dexamethasone on the incidence of ventilator-associated pneumonia in mechanically ventilated COVID-19 patients: a propensity-matched cohort study. 6/13/22. Scaravilli V. Crit Care.
- The role of extracorporeal membrane oxygenation in COVID-19. 6/6/22. Dalia AA. J Cardiothorac Vasc Anesth.
Newsletter Issue 136, June 27, 2022:
- Immune boosting by B.1.1.529 (Omicron) depends on previous SARS-CoV-2 exposure. 6/14/22. Reynolds CJ. Science.
Even in those triple vaccinated, Omicron (B.1.1.529) can evade immune defenses. These investigators studied triple mRNA-vaccinated healthcare workers (TVHCW) with diverse COVID histories. Before suffering Omicron infections, TVHCW showed robust in vitro blood antibody, B- and T-cell immunity to all variants of concern (VOC) EXCEPT Omicron. Prior Alpha infections singularly dampened Omicron response duration. Previously infection-naive TVHCW who then suffered Omicron infections showed boosted immune responses to all VOC EXCEPT Omicron. TVHCW with a history of prior COVID infection followed by Omicron infections had negligible immune boosting. Interestingly, a prior Wuhan Hu-1 infection abrogated Omicron immune defenses. Inability of Omicron to boost itself invites reinfection.
SAB Comment: It had been assumed that SARS CoV-2 infections would boost immune defenses against SARS-CoV-2, and that “hybrid immunity” (both vaccine and infection) would provide an even greater boost. This study shows that Omicron fails to boost immune responses in TVHCWs (remember-vaccine is Wuhan strain-based). The failure was especially profound if subjects had past Wuhan or Alpha infections termed, “hybrid immune-dampening.”
This study predicts: 1) Omicron re-infection is possible/probable, and 2) vaccination + the specific SARS CoV-2 strain (e.g., Wuhan, Alpha, Delta) infection history will shape (“imprint”) the strength and durability of responses.
Omicron produces poor antibody, T- and B-cell immunogenicity against itself, and predicts vaccines based purely upon Omicron-sequences may produce poor immunogenicity and protection unless paired with ancestral-sequence-based vaccine. - Neutralization Escape by SARS-CoV-2 Omicron Subvariants BA.2.12.1, BA.4, and BA.5. 6/22/22. Hachmann N. NEJM.
These authors evaluated the neutralizing antibody titer levels against the reference WA1/2020 (ancestral) isolate of SARS-CoV-2 along with Omicron subvariants BA.1, BA.2, BA.2.12.1, and BA.4 or BA.5 in 27 participants who had been vaccinated and boosted with messenger RNA vaccine BNT162b2 (Pfizer–BioNTech). As expected, the neutralizing antibody titer was lower by a factor of 6 to 21 against the Omicron variants compared with the response against the WA1/2020 isolate. As compared with the median neutralizing antibody titer against the BA.1 subvariant, the median titer against the other omicron subvariants was lower by a factor of 2.2 against the BA.2.12.1 subvariant and by a factor of 3.3 against the BA.4 or BA.5 subvariant. The authors also tested neutralizing antibodies in an additional 27 participants (only one of whom had ever been vaccinated) who had been infected with the BA.1 or BA.2 subvariant a median of 29 days earlier. As compared with the median titers against the BA.1 subvariant, the median titer was lower by a factor of 1.5 against the BA.2.12.1 subvariant and by a factor of 2.9 against the BA.4 or BA.5 subvariant. - Rapid, scalable assessment of SARS-CoV-2 cellular immunity by whole-blood PCR. 6/13/22. Schwarz M. Nature Biotechnology.
Low T-cell activation measurements to SARS-CoV-2 is predictive of COVID-19 breakthrough and need for revaccination. T-cell assays are difficult and rarely performed. These investigators developed fast, high-throughput quantitative PCR assays for T-cell activation. The tests stimulate whole-blood cells with SARS-CoV-2 antigens. Viral-specific T-cells secrete IFN-γ, which then stimulate monocytes to produce the cytokine CXCL10 mRNA which correlates and proved a proxy for SARS COV-2 antigen-specific T-cells activation. These assays may allow large-scale monitoring of both the magnitude and duration of functional T-cell immunity to SARS-CoV-2. In vulnerable populations, such screening may predict breakthrough infections and help prioritize revaccination strategies. - Nasal Spray of Neutralizing Monoclonal Antibody 35B5 Confers Potential Prophylaxis Against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Variants of Concern (VOCs): A Small-scale Clinical Trial. 6/6/22. Lin Y. Clin Infect Dis.
These investigators studied a nasal spray formulation of a SARS-CoV-2 neutralizing IgG1 monoclonal antibody (“35B5”). In 30 healthy, disease-naïve but vaccinated volunteers, nasal mucosal samples were assayed against spike proteins of the wild type (WT) and variants of concern (VOC) including Omicrib (B.1.1.529) at 0h and again at 12h-72h post-spray. Samples collected within 24 hours following a single spray neutralized WT and all VOC. Protection efficacy dropped to 60% and 20% at 48h and 72h, respectively. At a time where most monoclonals have lost efficacy especially against Omicron, the 35B5 nasal spray formulation may enhance SARS-CoV-2 prevention especially in unvaccinated and high-risk populations.
SAB Comment: This novel means of prevention at upper airway sites of viral introduction will need assessment in large-scale clinical trials. However, if proven effective, easy-to-administer intranasal monoclonals against VOC including Omicron would fill an unmet clinical need for those anticipating potential exposure including medical settings. - Below you will find a list of additional articles and resources selected for the IARS COVID-19 Resource website, all of which include a summary with major takeaways and are searchable by topic:
- BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection. 6/17/22. Cao Y. Nature.
- Effects of Previous Infection and Vaccination on Symptomatic Omicron Infections. 6/15/22. Altarawneh HN. N Engl J Med.
- Effectiveness of Homologous and Heterologous Covid-19 Boosters against Omicron. 5/25/22. Accorsi EK. N Engl J Med.
- Risk of long COVID associated with delta versus omicron variants of SARS-CoV-2. 6/18/22. Antonelli M. Lancet.
Newsletter Issue 135, June 22, 2022:
- Safety and immunogenicity of a live-attenuated influenza virus vector-based intranasal SARS-CoV-2 vaccine in adults: randomised, double-blind, placebo-controlled, phase 1 and 2 trials. 6/1/22. Zhu F. Lancet Respir Med.
In this randomized, single-center, double-blind, placebo-controlled trial in healthy COVID-naïve adults, these investigators published the first intranasal vaccine results in humans. The Phase 1 (n=63 subjects), phase 2 (n=724) and phase 2-extension (n=297) trial arms administered a live-attenuated influenza virus vector-based SARS-CoV-2 spray (“dNS1-RBD”), 2 doses, 14 days apart. Peripheral blood showed both weak cellular (40-46% conversion) and IgG and IgA humoral responses (10-13% conversion). Secretory IgA (nasopharynx) conversions and concentrations were also weak. “dNS1-RBD” was well tolerated and without serious adverse effects (0-12 months). Despite apparent “weak” effects, this vaccine may be effective in a real-world, phase-3 trial, which is ongoing. - Limited cross-variant immunity from SARS-CoV-2 Omicron without vaccination. 5/18/22. Suryawanshi RK. Nature.
This complex study applies basic laboratory research to answer the question whether widespread infections with the Omicron variant will eventually lead to herd immunity and end the COVID-19 epidemic. After creating mouse models susceptible to various SARS CoV-2 variants (WA1, Delta and Omicron), the authors compared their impact on respiratory tract pathology and immune markers and showed that Omicron infections were less severe and resulted in a diminished inflammatory response. When collecting convalescent serum from mice and humans, a diminished humoral immune response to Omicron infections compared to other variants indicated limited cross-variant neutralization induced by Omicron in mice and humans. Sera collected from vaccinated individuals experiencing breakthrough infections with Omicron developed higher neutralization titers against all variants, demonstrating that Omicron enhances pre-existing immunity but lacks protection against non-Omicron variants in the unvaccinated. Sera collected from vaccinated individuals experiencing breakthrough infections with Omicron developed higher neutralization titers against all variants, demonstrating that Omicron enhances pre-existing immunity but lacks protection against non-Omicron variants in the unvaccinated. - Virological characteristics of the SARS-CoV-2 Omicron BA.2 spike. 5/14/22. Yamasoba D. Cell.
Authors review the mechanism of infection of all SARS-CoV-2 variants and studied infectivity and immune escape of BA.2, which has over 30 mutations compared with the Wuhan strain. Data suggest that similar to BA.1, BA.2 is highly resistant to antisera induced by vaccination or infection with other SARS-CoV-2 variants as well as three therapeutic antiviral antibodies. BA.2 has a 1.4-fold higher effective reproductive rate than BA.1 as well as higher fusion potential and pathogenic potential. BA.2 was 4-fold more resistant to BA.1-infected sera from convalescents without full vaccination. Multiple studies using convalescent human, hamster, and murine sera demonstrated that BA.1-induced humoral immunity is less effective against BA.2, but not vise versa. Virologic features and proposed mechanistic consequences are reviewed.
Newsletter Issue 134, June 13, 2022:
- Effectiveness of Paxlovid in Reducing Severe COVID-19 and Mortality in High Risk Patients. 6/2/22. Najjar-Debbiny R. Clin Infect Dis.
This retrospective cohort study examines the effectiveness of Paxlovid on COVID-19 progression and mortality in a real life, uncontrolled setting, during the early Omicron phase using an Israeli data base of 180,000. Among 4737 patients receiving Paxlovid within 5 days of a positive PCR test and having at least one risk factor, a significant decrease in progression to severe COVID-19 and mortality occurred resulting in an HR of 0.54%, compared to full vaccination with an even more favorable outcome and an HR of 0.20. Paxlovid appears to be more effective in older and immunosuppressed patients, and patients with underlying neurological or cardiovascular disease. Vaccination remained the most effective means of preventing progression of the disease. Paxlovid had received an emergency release by the FDA based on pre-Omicron data obtained following the EPIC-HR controlled trial. This study proves its effectiveness in the Omicron era. - Baricitinib versus dexamethasone for adults hospitalised with COVID-19 (ACTT-4): a randomised, double-blind, double placebo-controlled trial. 5/26/22. Wolfe CR. Lancet Respir Med.
This trial aimed to compare the effectiveness of 2 immunomodulators, baricitinib and dexamethasone, given orally in combination with remdesivir, in preventing progression to mechanical ventilation or death in hospitalized patients with COVID-19. Between December 2020 and April 2021, it enrolled 1010 patients whose oxygen requirements ranged from supplemental to noninvasive mechanical ventilation at 67 sites. While there was no difference in mechanical ventilation-free survival by day 29 between these groups (odds ratio 1.01), treatment-related adverse events and adverse events in general, some life threatening, among patients receiving dexamethasone exceeded those receiving baricitinib significantly. The number needed to harm for one additional severe or life threatening adverse event with dexamethasone was 12·5, suggesting that immunomodulation should be tailored according to individual patient’s risk profile and other variables. - Below you will find a list of additional articles and resources selected for the IARS COVID-19 Resource website, all of which include a summary with major takeaways and are searchable by topic:
- Long COVID after breakthrough SARS-CoV-2 infection. 5/25/22. Al-Aly Z. Nat Med.
- Limited cross-variant immunity from SARS-CoV-2 Omicron without vaccination. 5/18/22. Suryawanshi RK. Nature.
- Virological characteristics of the SARS-CoV-2 Omicron BA.2 spike. 5/14/22. Yamasoba D. Cell.
- Safety of COVID-19 Vaccination in US Children Ages 5-11 Years. 5/18/22. Hause AM. Pediatrics.
- Protection and Waning of Natural and Hybrid Immunity to SARS-CoV-2. 5/25/22. Goldberg Y. N Engl J Med.
- Safety and immunogenicity of a live-attenuated influenza virus vector-based intranasal SARS-CoV-2 vaccine in adults: randomised, double-blind, placebo-controlled, phase 1 and 2 trials. 6/1/22. Zhu F. Lancet Respir Med.
Newsletter Issue 133, June 6, 2022:
- Health outcomes in people 2 years after surviving hospitalisation with COVID-19: a longitudinal cohort study. 5/14/22. Huang L. Lancet Respir Med.
Outcome of 1,192 patients 6 months, 12 months, and 2 years posthospitalization for COVID-19 between 1/7/20 and 5/29/20 in Wuhan, China is the topic of this longitudinal observational study. In addition, subjects were matched 1:1 by age, sex, and comorbidities, to a data set, created at the 12-month stage of 3,383 community-dwelling adults without previous SARS-CoV-2 infection. A subset of approximately 350 patients received pulmonary function studies and high resolution chest CT scans, with further CT scans only for those with abnormal lung images. All survivors underwent physical exam, routine labs, six-minute walk test and multiple standard questionnaires. Highlights of multiple results at 2 years: 55% had at least one symptom, 14% had dyspnea by the modified British Medical Research Council scale and 12% had anxiety/depression by the Health Related Quality of Life assessment. In addition, 89% had returned to their original work. Compared to controls, those who had respiratory support during hospitalization had reduced diffusion capacity, reduced residual volume, and reduced total lung capacity. The authors conclude further study is needed regarding the possibility of emerging pulmonary fibrosis. - Surgical Triage and Timing for Patients with COVID: A Guidance Statement from the Society of Thoracic Surgeons. 5/20/22. Grant MC. Ann Thorac Surg.
In this statement, universal preoperative testing for SARS-CoV-2, preferably PCR, is recommended. Further guidance, to be individualized, is dictated by a combination of procedure urgency, COVID-19 illness severity, and the present tier of the institution’s COVID-19 response, as outlined in the full publication. Example: For asymptomatic patients screening positive for SARS-CoV-2, it is recommended to defer non-urgent cardiac procedures at approximately 4-8 weeks. Patients with a procedural delay greater than 90 days from a positive test result should undergo repeat preoperative COVID-19 testing to screen for potential reinfection; prior testing following infection increases false positives, particularly with PCR. There is no convincing evidence that the type of anesthetic, airway management, or the use of regional anesthesia is associated with more favorable postoperative outcomes following recent COVID-19. - Below you will find a list of additional articles and resources selected for the IARS COVID-19 Resource website, all of which include a summary with major takeaways and are searchable by topic:
- The Influence of the COVID-19 Pandemic on Intensivists’ Well-Being: A Qualitative Study. 5/14/22. Vranas KC. Chest.
- Thromboembolic prevention and anticoagulant therapy during the COVID-19 pandemic: updated clinical guidance from the anticoagulation forum. 5/17/22. Barnes GD. J Thromb Thrombolysis.
- Venous or arterial thrombosis and deaths among COVID-19 cases: a European network cohort study. 5/16/22. Burn E. Lancet Infect Dis.
Newsletter Issue 132, May 23, 2022:
- Stress-Related Disorders of Family Members of Patients Admitted to the Intensive Care Unit With COVID-19. 4/25/22. Amass T. JAMA Internal Med.
This is a prospective US multicenter observational study of symptoms of stress-related disorder (PTSD) in 316 family members of patients admitted to the ICU for COVID-19 between February and July 2020. Questionnaires included the short-form Impact of Events Scale 6 (IES-6) for posttraumatic stress disorder (PTSD) and the Hospital Anxiety and Depression Scale (HADS). Screening for PTSD, anxiety and depression was positive in 64%, 45% and 31% of respondents at 3 months and 48%, 34% and 25% at 6 months. Mean IES-6 scores were higher in participants who were female or Hispanic and lower in those with graduate school experience. Respondents with higher IES-6 scores exhibited more distrust of practitioners. The authors cited pre-pandemic studies exhibiting an average incidence of PTSD of 30% in family members. They postulated that COVID-19 ICU visitation restrictions may inadvertently generate a secondary public health crisis through an epidemic of stress-related disorders in families.
SAB Comment: These data were obtained during the first COVID-19 surge in early 2020 and may not represent the incidence of stress-related family disorders in subsequent months or currently. Nonetheless, this study suggests that with the current emphasis on practitioner burnout, attention does need to be given to the impact on family members as well. - Hypercoagulability, endotheliopathy, and inflammation approximating 1 year after recovery: Assessing the long-term outcomes in COVID-19 patients. 4/28/22. Fan BE. Am J Hematol.
Sustained hypercoagulability and endotheliopathy have been shown to be present in convalescent COVID-19 patients for up to 4 months after recovery. This study shows that in a significant number of the 39 patients studied with documented prior infection, hypercoagulability, endothelial dysfunction, and inflammation are still detectable approximately 1 year after recovery. D-dimer, Factor VIII, Thrombin generation (Thromboscreen), vWF:Ag, ICAM-1, IL-6 and C-reactive protein were all noted to be elevated in portions (8-49%) of the previously infected patients compared with control patients. Subgroup analysis stratifying patients by COVID-19 severity and COVID-19 vaccination preceding SARS-CoV-2 infection did not show statistically significant differences.
SAB Comment: These observations are noteworthy. However, further study is required to evaluate indications for screening in larger populations, their relationship to delayed complications and ongoing hemostatic management, and whether or how we should modify our perioperative care for post-COVID patients presenting for elective or urgent surgery in the future. - Below you will find a list of additional articles and resources selected for the IARS COVID-19 Resource website, all of which include a summary with major takeaways and are searchable by topic:
- Thrombotic and bleeding events, mortality, and anticoagulant use among 546,656 hospitalized patients with COVID-19 in the United States: a retrospective cohort study. 4/30/22. Deitelzweig S. J Thromb Thrombolysis.
- American Society of Hematology living guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID-19: January 2022 update on the use of therapeutic-intensity anticoagulation in acutely ill patients. 5/3/22. Cuker A. Blood Adv.
Newsletter Issue 131, May 16, 2022:
- Remdesivir and three other drugs for hospitalised patients with COVID-19: final results of the WHO Solidarity randomised trial and updated meta-analyses. 5/5/22. WHO Solidarity Trial Consortium. Lancet.
Among several “repurposed antivirals” studied in this large 2-year trial, remdesivir emerged as the most promising and Solidarity is the only trial large enough to assess its effects on mortality. This final report subdivides cohorts by degrees of respiratory distress (not on oxygen, receiving oxygen, ventilated) at the time of randomization. Confirming the results of the placebo-controlled ACTT-1 trial which assessed length of stay, remdesivir had no effect on patients already ventilated but had a small effect on mortality in patients on oxygen breathing spontaneously (14·6% versus 16·3%; RR 0·87 [0·76–0·99], p=0·03). These findings are consistent with other reports listed by the COVID-Network Meta-Analysis initiative, a collaborative effort by the WHO and Cochrane.
SAB Comment: This report brings closure to a 2-year effort to assess the benefit of remdesivir in COVID-19 disease and highlights COVID-NMA as a resource for further study and research. - Early prolonged prone position in noninvasively ventilated patients with SARS-CoV-2-related moderate-to-severe hypoxemic respiratory failure: clinical outcomes and mechanisms for treatment response in the PRO-NIV study. 4/30/22. Musso G. Crit Care.
In a very detailed study, these Italian authors evaluated the utility of awake prone positioning (PP) in 81 proned and 162 non-proned COVID-19 patients with moderate to severe respiratory failure requiring non-invasive ventilation (NIV). Early prolonged PP (average 12 hours/day) was feasible and was associated with clinical benefits: NIV failure in 17% of PP patients versus 43% of controls, intubation in 11% of PP patients vs 30% of controls, and death in 12% of PP patients versus 36% of controls. Ventilatory, ultrasonographic and biochemical parameters were integrated to individually assess clinical improvements with PP therapy and to provide early signs of NIV failure.
SAB Comment: Though not randomized, this single-center study carefully matched treated and control patients and performed a myriad of statistical and lab/imaging studies which support their findings. Of note is the very long duration of proning in treated patients, exceeding that in other studies of awake PP. - Below you will find a list of additional articles and resources selected for the IARS COVID-19 Resource website, all of which include a summary with major takeaways and are searchable by topic:
- Delayed intubation is associated with mortality in patients with severe COVID-19: A single-centre observational study in Switzerland. 4/29/22. Le Terrier C. Anaesth Crit Care Pain Med.
- Neurologic Manifestations of Severe Acute Respiratory Syndrome Coronavirus 2 Infection in Hospitalized Patients During the First Year of the COVID-19 Pandemic. 4/25/22. Cervantes-Arslanian AM. Critical Care Explorations.
- Effectiveness of a COVID-19 Additional Primary or Booster Vaccine Dose in Preventing SARS-CoV-2 Infection Among Nursing Home Residents During Widespread Circulation of the Omicron Variant – United States, February 14-March 27, 2022. 5/5/22. Prasad N. MMWR Morb Mortal Wkly Rep.
- Comparative effectiveness over time of the mRNA-1273 (Moderna) vaccine and the BNT162b2 (Pfizer-BioNTech) vaccine. 5/2/22. Islam N. Nat Commun.
Newsletter Issue 130, May 4, 2022:
- Clinical characteristics with inflammation profiling of long COVID and association with 1-year recovery following hospitalisation in the UK: a prospective observational study. 4/23/22. The PHOSP-COVID Collaborative Group. Lancet Resp Med.
In this British prospective, longitudinal, cohort study, the proportion of adult COVID-19 patients reporting ongoing symptoms was virtually unchanged between 5 months (74.5% of n=1,965) and 1 year (70.1% of n=807) after hospital discharge. Data was gathered using questionnaires and physiologic testing. Female sex, obesity, and mechanical ventilation for COVID-19 were risk factors for poor recovery. Several inflammatory mediators including IL-6 were increased in individuals with the most severe physical, mental health, and cognitive impairments compared with others. Because data was collected on individuals discharged up to April 18, 2021, the numbers of one-year follow-ups are significantly lower than those 5 months post discharge. - Below you will find a list of additional articles and resources selected for the IARS COVID-19 Resource website, all of which include a summary with major takeaways and are searchable by topic:
- Influence of sex on development of thrombosis in patients with COVID-19: From the CLOT-COVID study. 4/7/22. Yamashita Y. Thromb Res.
- Clinical characteristics, physiological features, and outcomes associated with hypercapnia in patients with acute hypoxemic respiratory failure due to COVID-19—insights from the PRoVENT-COVID study. 3/27/22. Tsonas AM. J Crit Care.
- Comparing COVID-19-related hospitalization rates among individuals with infection-induced and vaccine-induced immunity in Israel. 4/23/22. Waxman JG. Nat Commun.
- Assessment of T-cell Reactivity to the SARS-CoV-2 Omicron Variant by Immunized Individuals. 4/22/22. De Marco L. JAMA Netw Open.
- Effectiveness of mRNA-based vaccines during the emergence of SARS-CoV-2 Omicron variant. 4/27/22. Sharma A. Clin Infect Dis.
Newsletter Issue 129, April 25, 2022:
- Multisystem Involvement in Post-Acute Sequelae of Coronavirus Disease 19. 12/24/21. Novak P. Ann Neurol.
This retrospective study evaluated 9 patients who presented consecutively with chronic fatigue, brain fog, and orthostatic intolerance 10 months following mildly symptomatic COVID-19 infection. Controls included patients with postural orthostatic, tachycardia syndrome (POTS) and healthy participants. Analyzed data included surveys, autonomic assessments (Valsalva maneuver, deep breathing, sudomotor, and tilt tests), cerebrovascular (cerebral blood flow velocity monitoring in middle cerebral artery), respiratory (capnography monitoring), and neuropathic testing (skin biopsies for assessment of small fiber neuropathy) as well as inflammatory/autoimmune markers. Findings for the patients with “post-acute sequelae of coronavirus disease 19” (PASC or Long COVID) included (1) cerebrovascular dysregulation with persistent cerebral arteriolar vasoconstriction; (2) small fiber neuropathy and related dysautonomia; (3) respiratory dysregulation; and (4) chronic inflammation. - Effect of Antiplatelet Therapy on Survival and Organ Support-Free Days in Critically Ill Patients With COVID-19: A Randomized Clinical Trial. 3/22/22. REMAP-CAP Writing Committee for the REMAP-CAP Investigators. JAMA.
This international RCT randomized 1,557 critically ill COVID-19 patients to aspirin or a P2Y12 inhibitor or no antiplatelet therapy and found no difference in organ support-free days up to 21 days. There was no improvement in survival with antiplatelet therapy. Those randomized to antiplatelet therapy had significantly increased bleeding. This study provides important data for frontline clinicians, and suggests that antiplatelet agents should not be used in critically ill COVID-19 patients.
SAB Comment: The accompanying editorial summarizes the prior RCTs for antiplatelet demonstrating no benefit in noncritically ill patients with COVID-19. - Fourth Dose of BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Setting. 4/13/22. Magen O. N Engl J Med.
The authors analyzed Israeli data from 182,122 recipients of a fourth vaccine dose, aged over 60 compared with individually matched controls who had received only a third dose at least 4 months earlier. Fourteen to 30 days after the fourth dose effectiveness was 52% against a positive PCR, 61% against symptomatic COVID-19, 72% against COVID-19-related hospitalization, 64% against severe COVID-19, and 76% against COVID-19-related death. Outcomes between groups began to diverge 7 days after the fourth vaccine dose. Because of the recent implementation of fourth vaccine administration, reported outcomes thus far are short-term.
SAB Comment: This study differs from a previously highlighted study from Israel regarding short-term outcomes after a fourth vaccine dose by individually pairing recipients with matched controls and by reporting more outcomes. - Additional articles and resources selected for the IARS COVID-19 Resource website and searchable by topic:
- Prospective Evaluation of Autonomic Dysfunction in Post-Acute Sequela of COVID-19. 4/5/22. Jamal SM. J Am Coll Cardiol.
- Effectiveness of COVID-19 mRNA Vaccination in Preventing COVID-19-Associated Hospitalization Among Adults with Previous SARS-CoV-2 Infection – United States, June 2021-February 2022. 4/14/22. Plumb ID. MMWR Morb Mortal Wkly Rep.
- Cardiac Complications After SARS-CoV-2 Infection and mRNA COVID-19 Vaccination – PCORnet, United States, January 2021-January 2022. 4/7/22. Block JP. MMWR Morb Mortal Wkly Rep.
- Auxora vs. placebo for the treatment of patients with severe COVID-19 pneumonia: a randomized-controlled clinical trial. 4/9/22. Bruen C. Crit Care.
- Association of Early Aspirin Use With In-Hospital Mortality in Patients With Moderate COVID-19. 3/24/22. Chow JH. JAMA Netw Open.
Newsletter Issue 128, April 20, 2022:
- Dysfunctional breathing diagnosed by cardiopulmonary exercise testing in ‘long COVID’ patients with persistent dyspnoea. 3/31/22. Frésard I. BMJ Open Respir Res.
Cardiopulmonary exercise testing (CPET) identifies mechanisms of dyspnea by simultaneously evaluating cardiovascular adaptation, ventilation and gas exchange through exercise. Dysfunctional breathing (DB) with normal PaCO2 and V̇E/V̇CO2 has been described in long COVID, particularly erratic ventilation with wide, irregular variations of tidal volume and breathing frequency over the progression of effort during CPET. DB evaluated by CPET occurred in 15 of the 51 COVID patients complaining of dyspnea. DB was associated with younger age and previous mild/moderate acute COVID and was present >200 days following infection. DB without hyperventilation with erratic breathing and deep sighs may also explain persisting dyspnea in long COVID patients. The pathophysiology is unknown. A prompt diagnosis is needed in order to offer specific respiratory training. - Trajectories of Neurologic Recovery 12 Months After Hospitalization for COVID-19: A Prospective Longitudinal Study. 3/22/22. Frontera JA. Neurology.
This study reports the results of telephone psychological testing and interviews of 294 patients 12 months after symptom onset of moderately severe to severe COVID-19 infection. Patients were tested to assess functional status and cognition and given structured interviews to determine presence of anxiety, depression, fatigue and sleep impairment. Abnormal scores on cognitive testing persisted in 50% of patients without a pre-COVID history of cognitive abnormalities, irrespective of the presence or absence of a neurological complication during hospitalization. Rates of abnormal cognition were substantially higher than rates of abnormalities in other domains such as activities of daily living, anxiety, depression, fatigue or sleep. Between the 6- to 12-month evaluations, the majority of patients did not have improvements in functional status or activities of daily living; however, there were significant improvements in cognition and anxiety scores. - Intravenous methylprednisolone pulses in hospitalised patients with severe COVID-19 pneumonia, A double-blind, randomised, placebo-controlled trial. 4/1/22. Salvarani C. Eur Respir J.
In this multicenter, randomized, double-blind, placebo-controlled trial, 304 hospitalized COVID-19 patients received either 3 boluses of 1 g of methylprednisolone intravenously daily for 3 days or placebo in addition to standard dexamethasone. The key outcome was overall survival in days, discharge without oxygen / ETT. The study group included patients who had < 5 days of symptoms, PaO2: FiO2>200 with O2 and C-reactive protein > 5. Outcomes were similar in both groups including % discharged without O2, duration of hospital or ICU stay, death or adverse reactions. Pulsed methylprednisolone in addition to dexamethasone was safe but redundant to treat hyper-inflammation. - Factors for success of awake prone positioning in patients with COVID-19-induced acute hypoxemic respiratory failure: analysis of a randomized controlled trial. 3/29/22. Ibarra-Estrada M. Crit Care.
Although awake prone positioning (APP) may be helpful, identifying which COVID-19 patients benefit is key. This multicenter, randomized controlled trial from Mexico of 941 COVID-19 patients with respiratory failure requiring high flow nasal cannula (HFNC) between May 2020 and January 2021 identified factors associated with success of APP in preventing intubation. Predictors of APP success in these patients that already required HFNC to maintain SpO2 over 91% included an APP duration at least 8 hours per day, a respiratory rate at enrollment below 26, and improvement in objective measurements (ROX and lung ultrasound) in response to APP. The number needed to treat to prevent intubation was 8.
SAB Comment: This is a post hoc analysis of a meta-trial featured previously in this newsletter. Though some studies do not show a benefit from APP for patients requiring simple nasal oxygen and using APP for shorter periods of time, this study clearly shows that patients with more severe respiratory failure may benefit, especially with longer APP times. Maintaining the prone position in sick, awake patients is difficult. Authors discuss their approach to this challenge.
Newsletter Issue 127, April 11, 2022:
- Protection by a Fourth Dose of BNT162b2 against Omicron in Israel. 4/5/22. Bar-On YM. N Engl J Med.
In early January 2022, a fourth Pfizer vaccination dose was offered to persons older than 60 years who had received their third shot at least 5 months previously. This study compares outcomes for 623,355 recipients with 628,966 persons also eligible for the 4th shot. Protection from infection during the ongoing Omicron wave peaked at 4 weeks after 4th shot by a factor of 2 compared with controls, and waned afterward. However, protection from severe disease was greater by a factor of 3.5 and remained durable for the 6 weeks of the study. - Association of COVID-19 Vaccination in Pregnancy With Adverse Peripartum Outcomes. 3/24/22. Fell DB. JAMA.
Association of SARS-CoV-2 Vaccination During Pregnancy With Pregnancy Outcomes. 3/24/22. Magnus MC. JAMA.
These two retrospective cohort studies address the safety of mRNA vaccines for COVID-19, administered during the last 2 trimesters of pregnancy. Magnus compares 157,521 pregnancies in Norway and Sweden, 18% (28,506) of which underwent vaccination between January 2021 and January 2022. There was no difference in the incidence of preterm birth, stillbirth, small for gestational age babies, decreased Apgar scores and NICU admissions. Fell compares 30,115 unvaccinated and 44,815 pregnancies vaccinated after birth, in Canada (from December 2020 to September 2021) to 22,660 (23%) pregnancies where vaccination occurred mostly in the third trimester. There was no difference in postpartum hemorrhage, chorioamnionitis, cesarean delivery, decreased Apgar scores and NICU admission. Both studies made adjustments for multiple covariants, which produced some differences between the characteristics of the vaccinated and unvaccinated groups. The authors conclude it is safe to administer mRNA vaccines in the second and third trimesters of pregnancy. - SARS-CoV-2 Placentitis and Intraparenchymal Thrombohematomas Among COVID-19 Infections in Pregnancy. 3/21/22. Huynh A. JAMA Netw Open.
This research letter describes a retrospective review, with clinical correlation, of 47 placentas infected with SARS-CoV-2 between January 2020 and November 2021. Placentas from 2021 were considered to represent predominantly Delta infection, and were compared to those of 2020. All placentas displayed syncytiotrophoblast necrosis, perivillous fibrin, and intervillositis, but, of the 39 cases from 2021, 29 also had intraparenchymal thrombohematomas. Stillbirth occurred in 72% of patients with thrombohematomas, whereas 17 of 18 placentas without thrombohematomas were associated with live births. Thrombohematomas could be observed on ultrasound.
SAB Comment: This study is of value for its hypotheses-generating potential, as well as to note that all placentas except one came from unvaccinated patients. - Additional articles and resources selected for the IARS COVID-19 Resource website and searchable by topic:
- Factors for success of awake prone positioning in patients with COVID-19-induced acute hypoxemic respiratory failure: analysis of a randomized controlled trial. 3/29/22. Ibarra-Estrada M. Crit Care.
- Intravenous methylprednisolone pulses in hospitalised patients with severe COVID-19 pneumonia, A double-blind, randomised, placebo-controlled trial. 4/1/22. Salvarani C. Eur Respir J.
- High-Flow Nasal Cannula Oxygen versus Non-Invasive Ventilation in Subjects with COVID-19: A Systematic Review and Meta-analysis of Comparative Studies. 3/23/22. Beran A. Respir Care.
- Early Outpatient Treatment for Covid-19 with Convalescent Plasma. 3/30/22. Sullivan DJ. N Engl J Med.
Newsletter Issue 126, April 4, 2022:
- SAB Comment: The following four peer-reviewed vaccine studies add to our overall understanding of mRNA vaccines. Specifically, boosters appear to be very safe; children benefit from vaccination, especially reducing critical COVID; and vaccination and boosters are excellent at preventing severe disease (invasive ventilation and death) even in older people with comorbidities and even during the Omicron phase of the pandemic.
- Boosters reduce in-hospital mortality in patients with COVID-19: An observational cohort analysis. 3/22/22. Mielke N. Lancet Reg Health Am.
This data-rich multicenter observational cohort study of 8,232 adult US COVID-19 patients hospitalized between 8/12/21 and 1/20/22 compared demographic, clinical, and outcome variables in those fully vaccinated and boosted (FV&B), 6%, fully vaccinated (FV), 29%, and unvaccinated (UV), 65%. Although a small number, FV&B patients with breakthrough COVID-19 had lower in-hospital mortality (7.1%) than those FV (10.3%) and UV (12.8%), despite being older and higher risk at baseline. Better outcomes for the FV&B were also found in subgroup analyses of patients older than 65 years and those requiring ICU care; although as expected mortality was higher. - Effectiveness of mRNA Vaccination in Preventing COVID-19-Associated Invasive Mechanical Ventilation and Death – United States, March 2021-January 2022. 3/24/22. Tenforde MW. MMWR Morb Mortal Wkly Rep.
To better evaluate vaccine effectiveness (VE) of both mRNA vaccines in preventing COVID-19 invasive mechanical ventilation (IMV) and death, these authors from 21 US centers used a case control design to study 1440 COVID-19 patients between March 2021 and January 2022. Though vaccinated patients were older, had more comorbidities (especially immunocompromising conditions) than unvaccinated patients, receiving 2 or 3 doses of an mRNA vaccine was associated with a 90% reduction in risk for COVID-19 IMV or death. Protection of 3 mRNA vaccine doses during the period of Omicron predominance was 94%. The authors conclude that COVID-19 mRNA vaccines provide strong protection against severe COVID-19 resulting in respiratory failure or death. - BNT162b2 Protection against the Omicron Variant in Children and Adolescents. 3/30/22. Price AM. N Engl J Med.
In this multicenter study conducted in 23 US states, the authors used a case-control, test-negative design to assess 2-dose BNT162b2 (Pfizer) vaccine effectiveness in preventing hospitalization and critical COVID-19 in 5- through 11- and 12- through 18-year-old patients. Case patients with COVID (n=1185) were compared to control patients (n=1627). Three fourths of subjects were unvaccinated. During the Omicron period, vaccine effectiveness for 12- through 18-year-old patients was 40% against hospitalization, 79% against critical COVID-19, and 20% against noncritical COVID-19. During the Omicron period, vaccine effectiveness against hospitalization among children 5 to 11 years of age was 68%. Ninety-five percent confidence intervals were wide. These figures are lower than during the Delta wave, particularly among adolescents. - Safety and Efficacy of a Third Dose of BNT162b2 Covid-19 Vaccine. 3/23/22. Moreira ED Jr. N Engl J Med.
In this phase 3 industry-sponsored multinational trial, 5,081 participants received a third BNT162b2 (Pfizer) dose and 5,044 received placebo between 7/1/21 and 8/10/21. All had received dose 2 at least 6 months before (median 10.7 months) and almost half had coexisting conditions. After 2 months, among those without evidence of previous SARS-CoV-2 infection, COVID-19 with onset at least 7 days after dose 3 was observed in 6 participants in the vaccine group vs. 123 in the placebo group, a relative vaccine efficacy of 95.3%. Efficacy began at 7 days and was maximal at 14 days. None in either group was hospitalized for COVID-19, supporting ongoing protection from serious disease from the first 2 shots. Injection-site pain was the most frequently reported adverse event. No new safety concerns, including cases of myocarditis or pericarditis, were reported. Serious adverse events were reported by fewer participants in the vaccine group than in the placebo group (0.3% vs. 0.5%).
- Boosters reduce in-hospital mortality in patients with COVID-19: An observational cohort analysis. 3/22/22. Mielke N. Lancet Reg Health Am.
- Additional articles and resources selected for the IARS COVID-19 Resource website and searchable by topic:
- Dysfunctional breathing diagnosed by cardiopulmonary exercise testing in ‘long COVID’ patients with persistent dyspnoea. 3/31/22. Frésard I. BMJ Open Respir Res.
- Trajectories of Neurologic Recovery 12 Months After Hospitalization for COVID-19: A Prospective Longitudinal Study. 3/22/22. Frontera JA. Neurology.
- SARS-CoV-2 Placentitis and Intraparenchymal Thrombohematomas Among COVID-19 Infections in Pregnancy. 3/21/22. Huynh A. JAMA Netw Open.
Newsletter Issue 125, March 28, 2022:
- One-year pulmonary impairment after severe COVID-19: a prospective, multicenter follow-up study. 3/22/22. Faverio P. Respir Res.
In this Italian multicenter, prospective, observational study, 287 patients hospitalized for SARS-CoV-2 pneumonia were stratified by maximum ventilatory support (“oxygen only,” “continuous positive airway pressure” and “invasive mechanical ventilation”) and followed up at 12 months after discharge. At that time, reduced diffusion capacity for carbon monoxide and non-fibrotic interstitial lung abnormalities were common, particularly in older patients who had required higher ventilatory support. Twenty percent of patients showed a distance walked lower than expected, without differences between groups. No patients showed oxygen desaturation or required oxygen supplementation during the test, but mild dyspnea was reported by 35% of patients, again with no differences between groups, compared to 29% at 6-month follow-up. - COVID-19-Associated Croup in Children. 3/8/22. Brewster RCL. Pediatrics.
This article from Boston Children’s Hospital describes the change in croup incidence as the COVID-19 variant evolved. During the 22 month-long study, a total of 75 children infected with SARS-CoV-2 entered ER or hospital care due to croup. A 4-fold increase in croup admissions occurred after 12/4/2021 as Omicron became the dominant viral form. During the study, 9 patients were hospitalized, with a median length of stay of 1.7 days. Four patients were admitted to the ICU. No patients died or were endotracheally intubated. Decadron was administered to 97% and all children hospitalized received racemic-epinephrine. Comprehensive viral testing was not available, so viral co-infection could not be excluded. Although rare (75 cases in 100,000 population of children seeking hospital care), the increase in croup was statistically significant during the Omicron surge, according to these authors. - Additional articles and resources selected for the IARS COVID-19 Resource website and searchable by topic:
- Effects of SARS-CoV-2 on prenatal lung growth assessed by fetal MRI. 3/19/22. Stoecklein S. Lancet Respir Med.
- Associations of statin use with 30-day adverse outcomes among 4 801 406 US Veterans with and without SARS-CoV-2: an observational cohort study. 3/19/22. Wander PL. BMJ Open.
- A randomized double-blind placebo-controlled clinical trial of nitazoxanide for treatment of mild or moderate COVID-19. 2/28/22. Rossignol J. eClin Med.
Newsletter Issue 124, March 22, 2022:
- NIH Therapeutics Recommendations – Updated March 2, 2022
Based upon data generated during the Omicron variant surge, the NIH has revised its recommendations for antiviral therapeutics for nonhospitalized patients with mild-moderate COVID-19 at high risk of progression to severe disease. The drugs are ranked by order of preference. Click here to read the full NIH treatment guidelines.
- Neutralization of the SARS-CoV-2 Omicron BA.1 and BA.2 Variants. 3/16/22. Yu J. N Engl J Med.
Omicron has three major sublineages: BA.1, BA.2, and BA.3, each with common and unique mutations to evade neutralizing antibodies (NAbs). Recently, BA.2 is surging. These investigators compared NAbs against Wuhan and BA.1/BA.2 strains induced post-Pfizer vaccinations (primaries + booster, n=24) vs. BA.1 natural infection (n=8). Two weeks postbooster vaccinations, NAbs to each virus rose by 10-fold vs post-primary vaccination; BA.1 NAbs =1.4x BA.2. Two weeks post-BA1 infection, BA.1 and BA.2 titers were each 3x higher vs. postbooster values. Conclusion: BA1 infection confers higher cross-reactive BA.2 NAbs than vaccination alone and the BA.2 surge is from increased infectivity, not immunological escape.
SAB Comment: Neutralizing antibody titers were undetectable to both BA.1 and BA.2 six months postprimary series before receiving boosters. This emphasizes a critical need for the six-month booster to protect against variants. Seven of eight infected patients were previously vaccinated. The unvaccinated eighth rapidly became critically ill. - Efficacy of a Fourth Dose of Covid-19 mRNA Vaccine against Omicron. 3/17/22. Regev-Yochay G. N Engl J Med.
This research letter from Israel examines the immune response and vaccine efficacy to a 4th dose of mRNA vaccine in 274 young, healthy healthcare workers, compared to matched controls. This non-randomized study took place in January 2022 during an Omicron surge. All subjects were tested with RT-PCR weekly. The 4th vaccine dose increased the antibody levels and viral neutralization by a factor of 10, but vaccine efficacy was low and relatively high viral loads were found, suggesting those with positive RT-PCRs were infectious. The authors conclude that a 4th vaccination of healthy people may have only marginal benefits. Older and vulnerable populations were not assessed.
SAB Comment: Though a small study, this is hopefully the first of much more information on the utility of a second booster. Of note is the infectiousness of those who get Omicron, whether or not they received a 4th vaccine dose (masks may still play a role), and that data on an older population with more comorbidities has yet to be presented. - Palliative care consultation and end-of-life outcomes in hospitalized COVID-19 patients. 12/13/21. Cheruku SR. Resuscitation.
This is a multicenter analysis of end-of-life care for 3,227 adult patients who died from COVID-19 between March 2020 and March 2021 in US hospitals without resource constraints, based on registry data of the Society of Critical Care Medicine. Cardiopulmonary resuscitation was given to 10% of patients and not given to 90%; about 20% of both groups had a palliative care consultation. However, patients who received individualized comfort care measures rather than continuation of life-sustaining treatment were significantly more likely to have had palliative care consultation (43.2% v 8.5%). The authors suggest that palliative care consultation at the end-of-life may better align the needs and values of patients with their received care. - The Fragility of Statistically Significant Results in Randomized Clinical Trials for COVID-19. 3/18/22. Itaya T. JAMA Netw Open.
The objective of this study was to use the fragility index (FI) to evaluate the robustness of statistically significant findings from RCTs for COVID-19. Forty-seven English language articles published by August 7, 2021, involving 138,235 participants were included. In order to apply the index, studies must randomly assign patients 1:1 into 2 parallel groups and reported at least 1 binary outcome as significant in the abstract. In this study, many randomized clinical trials (RCTs) had a low FI, challenging confidence in the robustness of the results. The median was 4, meaning a change in outcome of only 4 participants was required to change the analysis findings from “statistically significant” (P<0.05) to not significant. In over half of the trials, the FI was less than 1% of sample size. Thirty-six were drug trials, with a median FI of 2.5. The most robust group contained 6 vaccine trials, with a median FI of 119. Authors state, “The fragility of RCT results should be considered before applying them to clinical settings,” and “Health care professionals and policy makers should not rely heavily on individual results of RCTs on COVID-19,” particularly small studies. - Additional articles and resources selected for the IARS COVID-19 Resource website and searchable by topic:
Newsletter Issue 123, March 14, 2022:
- The immunology and immunopathology of COVID-19. 3/10/22. Merad M. Science.
On the two-year anniversary of the COVID-19 pandemic, these authors summarize progress in understanding immune mechanisms that lead to clinical expression of acute symptomatic and asymptomatic disease. They also detail immune mechanisms in the symptom prolongation involved in “Long COVID syndrome” (aka, post-acute sequelae of SARS-CoV-2, PASC). Viral entry is traced from initial innate mechanisms to adaptive mechanisms followed by resultant pathophysiology. Risk factors and current therapies intersecting immune mechanisms are discussed.
SAB Comment: This up-to-date review is very well written, cites key references and provides a state-of-the-art understanding of the pathophysiology of SARS-CoV-2 infection and its consequences. As such it will also be helpful to readers who decide to read the original paper on whole genome sequencing reviewed below. - Whole genome sequencing reveals host factors underlying critical Covid-19. 3/7/22. Kousathanas A. Nature.
This basic research study sought to find disease mechanisms by comparing whole genome sequencing (WGS) in 7,491 critical COVID-19 cases vs. 48,400 controls in 224 international ICUs. Twenty-three independently validated variants predisposed to critical COVID-19 including genes involved in interferon signaling, leucocyte differentiation, and blood type secretor status. Multiple genes supported causal roles including myeloid cell adhesion molecules and coagulation factor F8, druggable targets. Conclusion: Though complex and requiring multiple genetic comparator controls, WGS is a highly efficient method to detect therapeutically relevant mechanisms in critical COVID-19 including failure to control viral replication and increased tendencies towards pulmonary inflammation and intravascular coagulation. - SARS-CoV-2 is associated with changes in brain structure in UK Biobank. 3/7/22. Douaud G. Nature.
This longitudinal study compares brain scans from the UK Biobank in 401 participants who had COVID-19 to 384 matched controls who were negative for SARS-CoV-2. The scans were done 38 months apart, and an average of 141 days post-COVID-19 diagnosis. Hundreds of brain-imaging derived phenotypes (IDPs) were measured and analyzed. Compared to controls, viral exposure was associated with parahippocampal and orbitofrontal grey matter reduction, greater changes in markers of tissue damage in regions functionally connected to the olfactory cortex, greater reduction in global brain size and larger cognitive decline between scans. The finding remained when patients hospitalized for COVID-19 were removed. The average percent of change compared to control (-0.2 to -2%) was considered moderate by the authors. The study is ongoing. - Prolonged unconsciousness is common in COVID-19 and associated with hypoxemia. 3/7/22. Waldrop G. Ann Neurol.
In this multi-center, retrospective follow-up of 795 ICU patients from two waves of the COVID pandemic, the median time to recovery of command-following (RCF) was 30 days following the initiation of mechanical ventilation. The study group was limited to those patients who had a Glasgow Coma Score < 6 on the 7th day of endotracheal intubation. Median time to RCF increased by 16 days for patients with at least one episode of PaO2 ≤55mmHg (p<0.001). Time to RCF increases with duration of hypoxemia after adjusting for known confounders including sedation and does so even among patients without brain imaging abnormalities. The authors state “These observations should be taken into account when making decisions about life-sustaining therapies.” - Mechanically ventilated patients shed high titre live SARS-CoV2 for extended periods from both the upper and lower respiratory tract. 3/1/22. Saud Z. Clin Infect Dis.
Secretions from 25 mechanically ventilated COVID patients at the University Hospital of Wales were tested for viral RNA and infectious virions in early 2021. One hundred seventeen samples (44 saliva, 32 subglottic above ETT cuff, 41 bronchoalveolar lavage [BAL]) showed extremely high rates of positive qPCR across all sample types, however live virus was most common in saliva (68%) and least common in BAL (32%). Average titers of live virus were highest in subglottic aspirates. SARS-CoV-2 shedding typically ceases beyond 10 days from symptom onset; however, 14/25 studied patients shed live virus for >20 days and one for 98 days. “This information is important for decision making around cohorting patients, de-escalation of PPE, and undertaking potential aerosol generating procedures.” - Waning 2-Dose and 3-Dose Effectiveness of mRNA Vaccines Against COVID-19–Associated Emergency Department and Urgent Care Encounters and Hospitalizations Among Adults During Periods of Delta and Omicron Variant Predominance — VISION Network, 10 States, August 2021–January 2022. 2/11/22. Ferdinands J. MMWR Morb Mortal Wkly Rep.
This CDC study examines mRNA vaccine effectiveness (VE) in 241,204 emergency department/urgent care (ED/UC) encounters and 93,408 hospitalizations across 10 states between August 26, 2021 and January 22, 2022. During both the Delta and Omicron periods, vaccine effectiveness (VE after receipt of a third dose was always higher than VE following a second dose; however, VE waned with increasing time since vaccination. During the Omicron-predominant period, mRNA vaccination was highly effective against the occurrence of both emergency department/urgent care (ED/UC) encounters (VE = 87%) and hospitalizations (VE=91%) within 2 months after a third dose, but effectiveness declined to 66% for prevention of ED/UC encounters by the 4th month, and 78% for hospitalizations.
SAB Comment: This study shows VE after a third dose of mRNA vaccine is excellent against Omicron, though it does wane somewhat with time. The takeaway is that all persons should stay up to date with recommended vaccinations. - Frequency of Adverse Events in the Placebo Arms of COVID-19 Vaccine Trials: A Systematic Review and Meta-analysis. 1/18/22. Haas JW. JAMA.
The authors conducted a meta-analysis to investigate the frequency of adverse events (AEs) in placebo groups of 12 COVID-19 vaccine trials that included more than 45,000 participants, equally divided between vaccine and placebo recipients. There were significantly more systemic AEs in vaccine recipients, especially after the 2nd dose. However, about one-third of placebo recipients experienced at least one AE and the placebo arms accounted for 76% and 52% of systemic AEs after the 1st and 2nd doses respectively. These “nocebo” responses included headache, fatigue, malaise and joint pain and were similar to responses in patients receiving vaccine especially after the 1st dose. The authors suggest that informing the public about nocebo responses could decrease anxiety about vaccine side effects and vaccine hesitancy. - Additional articles and resources selected for the IARS COVID-19 Resource website and searchable by topic:
- Outpatient Pulmonary Rehabilitation in Patients with Long COVID Improves Exercise Capacity, Functional Status, Dyspnea, Fatigue, and Quality of Life. 2/24/22. Nopp S. Respiration.
- Clinical severity of, and effectiveness of mRNA vaccines against, covid-19 from omicron, delta, and alpha SARS-CoV-2 variants in the United States: prospective observational study. 3/10/22. Lauring AS. BMJ.
- Covid-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. 3/6/22. Andrews N. N Engl J Med.
- Assessing clinically meaningful hypercoagulability after COVID-19 Vaccination: a longitudinal study. 3/7/22. Campello E. Thromb Haemost.
- Characteristics and Outcomes of COVID-19 Patients Supported by Venoarterial or Veno-Arterial-Venous Extracorporeal Membrane Oxygenation. 3/7/22. Haroun MW. J Cardiothorac Vasc Anesth.
- Tixagevimab and Cilgavimab (Evusheld) for Pre-Exposure Prophylaxis of COVID-19. 1/25/22. JAMA.
Newsletter Issue 122, March 7, 2022:
- Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19. 2/16/22. Hammond J. N Engl J Med.
This industry-sponsored, phase 2-3, double-blind, randomized, controlled trial treated patients with either nirmatrelvir and ritonavir (Paxlovid, n=1039) or placebo (n=1046). Patients were symptomatic less than 5 days, unvaccinated, not hospitalized at enrollment and at high-risk. At 28 days, combined hospitalizations and deaths were 88% lower in the treated group. No deaths were reported in the treated group. Fewer serious adverse events and adverse events leading to discontinuation occurred with nirmatrelvir and ritonavir than with placebo. The most frequent adverse events occurring more often in recipients of nirmatrelvir plus ritonavir were dysgeusia, diarrhea and vomiting.
SAB Comment: This is the definitive study on the use of Paxlovid. See highlights from the NIH therapeutic guidelines for possible drug interactions. - ‘I can’t cope with multiple inputs’: a qualitative study of the lived experience of ‘brain fog’ after COVID-19. 2/12/22. Callan C. BMJ Open.
The authors collected and analyzed comments from 50 patients describing their sensations living with neurocognitive dysfunction resulting from “long COVID.” Qualitative analysis revealed the following themes: rich descriptions of the experience of neurocognitive symptoms (especially executive function, attention, memory and language), accounts of how the illness fluctuated — and progressed over time; the profound psychosocial impact of the condition on relationships, personal and professional identity; self-perceptions of guilt, shame and stigma; strategies used for self-management; challenges accessing and navigating the healthcare system; and participants’ search for physical mechanisms to explain their symptoms.
SAB Comment: The analysis is supplemented with a series of direct quotes from some of the study subjects which may give valuable insight to clinicians caring for similar patients. - Risks of mental health outcomes in people with covid-19: cohort study. 2/16/22. Xie Y. BMJ.
This Veterans Administration study carefully documents the mental health outcomes of 153,848 COVID-19 survivors at one year. These outcomes were compared to two control groups, contemporaneous and pre-COVID. The risks of mental health disorders was substantial and spanned several disorder categories, including anxiety, depression, stress and adjustment disorders, opioid and other substance use disorders, cognitive decline and sleep disorders. The risks were evident even among those with COVID-19 who did not require hospital admission. The authors feel that tackling mental health disorders among survivors of COVID-19 should be a priority.
SAB Comment: The authors analyzed an enormous amount of data, and presented the results in a series of graphs and tables which succinctly and clearly summarize the scope of post-COVID mental health problems. An associated opinion by the senior author cautions against dismissing these long COVID mental health problems as psychosomatic. - Association of COVID-19 Acute Respiratory Distress Syndrome With Symptoms of Posttraumatic Stress Disorder in Family Members After ICU Discharge. 2/18/22. Azoulay E. JAMA.
This is a prospective 2020 cohort study in 23 French ICUs. Family members of patients with COVID-19 ARDS had a significantly higher prevalence of symptoms of posttraumatic stress disorder (PTSD), anxiety, and depression at 90 days after patients’ discharge from the ICU than family members of patients with non–COVID-19 ARDS. Three hundred and seven patients and 602 family members participated. Twenty-six percent of patients died before the relatives had the day-90 assessment, similar for patients with and without COVID-19. PTSD symptoms were significantly higher in families of patients who died from COVID-19 compared with non-COVID-19 ARDS. Non-COVID-19 ARDS and COVID-19 ARDS survivors had rates of symptoms that were not significantly different for PTSD, anxiety, or depression. Compared with family members, ICU survivors reported fewer PTSD symptoms. The discussion addresses outcome determinants. - Thromboprophylaxis in Patients with COVID-19. A Brief Update to the CHEST Guideline and Expert Panel Report. 2/15/22. Moores LK. Chest.
This article updates the CHEST guidelines for thromboprophylaxis for COVID-19. Briefly, they suggest full dose anticoagulation with heparin for acutely ill hospitalized patients not in the ICU who are at high risk for deep vein thrombosis and without high risk for bleeding. The remainder of hospitalized patients should be on prophylactic doses of heparin. They do not suggest intermediate doses of heparin. These guidelines are developed using a Delphi approach. In addition, they summarize the evidence supporting these conclusions. This concise report represents the most up-to-date guidelines and is useful for clinicians.
SAB Comment: This manuscript is based upon the evidence (RCTs) that have demonstrated improved mortality and decreased need for organ support with these protocols. The authors do take into account the implementation of these guidelines. - Noninvasive respiratory support for COVID-19 patients: when, for whom, and how? 1/15/22. Sullivan ZP. J Intensive Care.
This is a directed review of non-invasive respiratory support (NIRS) for patients with COVID-19 based upon an extensive evaluation of its literature with and without proning (57 citations). Under the NIRS rubric the authors include high flow nasal cannula (HFNC), continuous positive airway pressure (CPAP), and non-invasive ventilation (NIV) which predominantly implies bilevel positive airway pressure (BiPAP). They discuss the rationale and evidence for NIRS in enhancing outcome, particularly avoidance of invasive mechanical ventilation (IMV), in patients with COVID-19 but also compare its use in prior pandemics (SARS, MERS, H1N1) and other forms of acute respiratory failure. They use this to generate a COVID-19 NIRS decision algorithm that includes indications, contraindications, most appropriate form of NIRS, monitoring, steps to reduce health care worker viral exposure and predictors of failure. The authors conclude that judicious use of NIRS may provide an acceptable alternative to early IMV in COVID-19 patients with mild to moderate acute respiratory failure.
SAB Comment: This is an excellent overview that provides a practical summary of the different forms of NIRS, their historic and COVID-19 evidence basis, and a coherent step-wise clinical guideline to decision-making in their application and transition to IMV. As such it provides an extremely useful resource for providers who care for hospitalized COVID-19 patients. - Respiratory mucosal delivery of next-generation COVID-19 vaccine provides robust protection against both ancestral and variant strains of SARS-CoV-2. 2/18/22. Afkhami S. Cell.
Using both a human and chimpanzee adenoviral (Ad) vectored vaccine expressing three COVID antigens (spike-1, and internal/conserved nucleocapsid, and RNA-dependent-RNA-polymerase) these investigators showed marked protection in a murine model. Single-dose intranasal was much superior to IM immunization. Tripartite protective immunity was demonstrated in local and systemic antibody responses, mucosal tissue-resident memory T cells and mucosal-trained innate immunity. Intranasal immunization protected against the ancestral strain and two variants of concern (VOC): alpha (UK) and beta (S. African). Conclusion: Intranasal mucosal delivery of this Ad-vectored multivalent vaccine is a next-generation COVID-19 vaccine strategy to induce all-around mucosal immunity against current and future VOC.
SAB Comment: This scientific tour de force used a murine model to rigorously demonstrate the superior protective effects of their trivalent vaccine administered intranasally vs. IM. Human phase 1 trials have begun comparing immune responses to both the human and chimpanzee versions after aerosol delivery to mRNA-vaccinated humans (clinicaltrials.gov: NCT05094609). - Additional articles and resources selected for the IARS COVID-19 Resource website and searchable by topic:
- Newly diagnosed diabetes vs. pre-existing diabetes upon admission for COVID-19: Associated factors, short-term outcomes, and long-term glycemic phenotypes. 2/4/22. Cromer SJ. Journal of Diabetes and Its Implications.
- Association of SARS-CoV-2 Infection With Serious Maternal Morbidity and Mortality From Obstetric Complications. 2/7/22. Metz TD. JAMA.
- Effect of prone positioning on survival in adult patients receiving venovenous extracorporeal membrane oxygenation for acute respiratory distress syndrome: a systematic review and meta-analysis. 1/17/22. Papazian L. Intensive Care Med.
- Protection against the Omicron Variant from Previous SARS-CoV-2 Infection. 2/9/22. Altarawneh HN. N Engl J Med.
Newsletter Issue 121, February 28, 2022:
- Timing of elective surgery and risk assessment after SARS-CoV-2 infection: an update: A multidisciplinary consensus statement on behalf of the Association of Anaesthetists, Centre for Perioperative Care, Federation of Surgical Specialty Associations, Royal College of Anaesthetists, Royal College of Surgeons of England. 2/23/22. El-Boghdadly K. Anaesthesia.
This multidisciplinary update of the guidelines for surgery after SARS-CoV-2 infection comes from several United Kingdom medical organizations, focusing on the Omicron variant. The authors emphasize there is no data yet available regarding surgery after Omicron, and the previous need to wait 7 weeks to avoid any increase in risk stands. Avoiding surgery during active infection, vaccination, preoperative prevention measures, exercise, and hospital prevention measures are emphasized. Risk assessment with a tool such as Surgical Outcome Risk Tool v2 (SORT-2) is recommended along with an instructive example of how to calculate individual patient risk. The possibility that use of local or regional anesthesia may lower risk compared with general anesthesia is also discussed. - ASA/APSF joint statement on elective surgery/procedures and anesthesia for patients after COVID-19 infection. 2/2/22. ASA & APSF.
Data from 2020, before vaccine availability, revealed a relative 30-day mortality risk ratio of 2.3 to 3.1 compared to normal following elective surgery performed up to 7 weeks post COVID-19. To minimize postoperative complications, the ASA/APSF recommendation is to delay elective surgery in the unvaccinated for at least 7 weeks following documented COVID-19, and potentially longer if symptoms persist. Data are inconclusive for vaccinated patients and later variants. If surgery is scheduled over 90 days after a COVID-19 diagnosis, a new preoperative PCR test is recommended. Unless new symptoms occur, preoperative PCR testing is not recommended within 90 days of COVID-19, due to the potential for persistent positive tests. - Pulse oximeters’ measurements vary across ethnic groups: An observational study in patients with Covid-19 infection. 1/28/22. Crooks CJ. Eur Respir J.
This research letter from Great Britain addresses the accuracy of pulse oximetry, with regard to skin pigmentation, when oxygen saturation is low. From electronic records, 5374 arterial blood gas oxygen (ABG) saturation results were compared to pulse oximetry saturations obtained within 30 minutes of each other. The study was carried out between February 2020 and September 2021, involved 2997 patients who had suspected or confirmed SARS-CoV-2 and were not in the ICU. Ethnicity, reported as White, Black, Asian, or Mixed, was obtained from the record; the number of non-White patients was small. Pulse oximetry underestimated ABG-determined oxygen saturations greater than 95%, and overestimated low saturations. When ABG saturations were 85-89%, pulse oximetry was higher by 2.4% in Whites, 3.9% in Blacks, and 5.8% in Asians. The authors note greater pulse oximetry inaccuracy in southeast Asians as well as Blacks.
SAB Comment: The statistics here are presented as a reminder of limited accuracy of pulse oximetry, especially isolated measurements. Its value lies in providing trends with continuous monitoring. - Extracorporeal membrane oxygenation in coronavirus disease 2019: A nationwide cohort analysis of 4279 runs from Germany. 2/18/22. Friedrichson B. Eur J Anaesthesiol.
A country-wide review of ECMO used in 4,279 COVID-19 patients over an 18-month period ending September 2021 revealed an overall in-hospital mortality of 72% for VA ECMO (291/404) and 65.9% for VV ECMO (2,552/3,875). This contrasted with a previous report of 53% mortality. In those over 60 (43.2%, n=1848), hospital mortality rate was 72.7% for VA ECMO (n=172) and 77.6% for VV ECMO (n=1,301). For those under 60, mortality was 71.6% for VA ECMO (n=166) and 56.9% for VV ECMO (n=1251). Only 7.1% of 14 VV ECMO patients over 80 survived. Intracranial complications, cardiac arrest and renal failure were significant associated findings. Dialysis was associated with a 3-fold mortality. The authors conclude that, “An unconditional recommendation cannot be given for COVID-19,” particularly for those of advanced age. - SAB Comment: Although electrical impedance tomography (EIT) is not yet in widespread use, it is an established technology that provides easily repeatable non-invasive bedside organ function monitoring without radiation exposure. The following two studies demonstrate EIT’s usefulness in quantitating areas of lung collapse, dead space and perfusion, and thereby facilitate description and prediction of the benefit of interventions such as prone positioning in severe COVID-19 ARDS.
- Lung-Dependent Areas Collapse, Monitored by Electrical Impedance Tomography, May Predict the Oxygenation Response to Prone Ventilation in COVID-19 Acute Respiratory Distress Syndrome. 12/11/22. Cardinale M. Critical Care Medicine.
The authors conducted an experimental study to determine the correlation between lung-dependent area collapse calculated by electrical impedance tomography (EIT) and oxygenation response (>20% increase in PaO2/FiO2) to prone positioning. In studying 24 patients with severe COVID-19 ARDS, the authors found that dependent lung areas collapse of greater than 13.5% had a 94% positive predictive value for improved oxygenation with prone positioning. They also found a response prediction AUC of 0.94. - Effects of Prone Position on Lung Recruitment and Ventilation-Perfusion Matching in Patients With COVID-19 Acute Respiratory Distress Syndrome: A Combined CT Scan/Electrical Impedance Tomography Study. 2/11/22. Fossali T. Critical Care Medicine.
The authors conducted an experimental study to compare the physiologic effects of supine and prone positioning in 21 patients with severe COVID-19 ARDS (C-ARDS) using computed tomography (CT) scanning and electrical impedance tomography (EIT). They observed that prone positioning induced net alveolar recruitment (dorsal recruitment > ventral derecruitment) without change in compliance. This and other observations suggested that prone positioning decreased the potential for atelectrauma. EIT indicated a decrease in ventral dead space and improved ventilation-perfusion matching. The authors conclude that these physiologic responses may be associated with enhanced protective lung ventilation during prone positioning.
- Lung-Dependent Areas Collapse, Monitored by Electrical Impedance Tomography, May Predict the Oxygenation Response to Prone Ventilation in COVID-19 Acute Respiratory Distress Syndrome. 12/11/22. Cardinale M. Critical Care Medicine.
- Early Administration of Remdesivir and Intensive Care Unit Admission in Hospitalized Pregnant Individuals With Coronavirus Disease 2019 (COVID-19). 2/8/22. Eid J. Obstet Gynecol.
A single-center, retrospective study compared outcomes in 24 pregnant patients who received remdesivir initiated less than 7 days (mean 3 days) from onset of patient-reported symptoms with 17 pregnant patients who received remdesivir initiated 7 or more days from symptom onset (mean 9 days). Patients in the early group were less likely to be admitted to the ICU (21% vs 59%), or to progress to critical disease (12% vs 41%). Additionally, those in the early group had shorter hospital stays (5 days vs 11).
SAB Comment: This result is in line with the recent NIH recommendation that includes remdesivir treatment for COVID-19 patients with elevated risk but not requiring hospitalization or supplementary oxygen. See an overview of the NIH guidelines here. - Additional articles and resources selected for the IARS COVID-19 Resource website and searchable by topic:
- Association of COVID-19 Acute Respiratory Distress Syndrome With Symptoms of Posttraumatic Stress Disorder in Family Members After ICU Discharge. 2/18/22. Azoulay E. JAMA.
- Risks of mental health outcomes in people with covid-19: cohort study. 2/16/22. Xie Y. BMJ.
- Respiratory mucosal delivery of next-generation COVID-19 vaccine provides robust protection against both ancestral and variant strains of SARS-CoV-2. 2/18/22. Afkhami S. Cell.
- Noninvasive respiratory support for COVID-19 patients: when, for whom, and how?. 1/15/22. Sullivan ZP. J Intensive Care.
- Frequency of Adverse Events in the Placebo Arms of COVID-19 Vaccine Trials: A Systematic Review and Meta-analysis. 1/18/22. Haas JW. JAMA.
- Waning 2-Dose and 3-Dose Effectiveness of mRNA Vaccines Against COVID-19–Associated Emergency Department and Urgent Care Encounters and Hospitalizations Among Adults During Periods of Delta and Omicron Variant Predominance — VISION Network, 10 States, August 2021–January 2022. 2/11/22. Ferdinands J. MMWR Morb Mortal Wkly Rep.
Newsletter Issue 120, February 22, 2022:
SPECIAL EDITION: Mental Health and COVID-19
- SAB Comment: Due to increasing attention to and recognition of COVID’s negative psychologic impact on all healthcare workers [including physicians, nursing staff, nursing home caregivers, first responders, etc.], survivors and survivors’ families, the SAB is presenting the following articles as a current collation of relevant articles. Further information on this topic will be forthcoming.
- Anxiety, worry, and job satisfaction: effects of COVID-19 care on critical care anesthesiologists. 1/13/22. Siddiqui S. Can J Anaesth.
This correspondence describes the psychological impact of the COVID-19 pandemic on intensivists in late 2020. An online survey was sent to 1,400 mostly American intensivists, 21% of whom responded. Analysis of the results indicate that the COVID-19 pandemic is associated with a high incidence of generalized anxiety disorder (42%) and an increased sense of burnout among critical care anesthesiologists, particularly in females (73%) and younger physicians. This is balanced by enhanced job satisfaction and a sense of being respected and valued for contributions during the pandemic. Seventy-five percent felt that institutional wellness resources were unhelpful. - The Impact of the COVID-19 Pandemic on Mental Health, Occupational Functioning, and Professional Retention Among Health Care Workers and First Responders. 12/16/21. Hendrickson RC. Journal of General Intern Med.
The authors conducted an observational survey of 510 US health care workers and first responders at a single time-point between September 2020 and February 2021. The goal was to examine the relationships between COVID-19 occupational stressors and symptoms of posttraumatic stress disorder (PTSD), depression (and suicidality), insomnia and anxiety as well as functional impairment and likelihood to leave the current profession. Stressors included factors related to volume (intensity of patient suffering, long hours), demoralization (futile care, lack of personal protective equipment, inadequate support) and risk (to oneself or family). There was a direct relationship between the intensity of stressors (especially demoralization) and psychiatric symptoms (especially PTSD). Both were more intense in nurses than physicians and in emergency medical services than firefighters or police. More than half the health care workers reported that working in the pandemic decreased their likelihood of remaining in their current field. Based upon their data the authors suggest a number of strategies that could mitigate stressors, especially those related to demoralization. - Challenges for the Beleaguered Health Care Workforce During COVID-19. 1/27/22. Cutler DM. JAMA Forum.
This is a brief review of the COVID-19 pandemic-induced challenges to the health care workforce that have culminated in burnout and widespread job resignation. The latter has been particularly prevalent in low-wage occupations such as health aides and licensed practical nurses in home health care and nursing homes. Simultaneous increase or steady health care worker demand in the face of falling supply has resulted in wage increases, but still leaves hospitals and health care workers stressed because of inadequate patient coverage and stalled throughput. The author recommends strategies to reduce burnout, including vaccine mandates, continued reimbursement for telehealth, and billing simplification. - Mental health symptoms in family members of COVID-19 ICU survivors 3 and 12 months after ICU admission: a multicentre prospective cohort study. 2/1/22. Heesakkers H. Intensive Care Med.
To better understand the impact of a COVID-19 intensive care unit (ICU) admission on family members, this prospective cohort study from ICUs in 10 Dutch hospitals followed 197 family members of surviving ICU COVID-19 patients. Questionnaires were completed by family members at enrollment, 3 months and 12 months. Thirty-eight percent experienced at least one mental health symptom (anxiety, depression, or posttraumatic stress disorder) and 23% experienced two or more mental health symptoms, all significantly higher than baseline. Additionally, family members experienced a reduction in quality of life and an impaired work status. Clinicians, including non-ICU clinicians (e.g., general practitioners), should be aware of the high prevalence of mental health problems among family members of COVID-19 ICU patients, especially in family members with mental health symptoms prior to ICU admission.
Newsletter Issue 119, February 14, 2022:
- Long-term cardiovascular outcomes of COVID-19. 2/8/22. Xie Y. Nat Med.
In this Veteran’s Administration database study, COVID-19 survivors who survived 30 days or more after their first positive test, exhibited increased risks and 12-month burdens of cardiovascular diseases, including cerebrovascular disorders, dysrhythmias, inflammatory heart disease, ischemic heart disease, heart failure, thromboembolic disease and other cardiac disorders. Two key findings: (1) the risks and associated burdens were evident among those who were not hospitalized during the acute phase of the disease; (2) complications and associated burdens were correlated with the severity of the acute phase of COVID-19. This finding suggests that care pathways of people who survived the acute episode of COVID-19 should include attention to cardiovascular health and disease. Excellent description of methods accompanied by helpful tables.
SAB Comment: This VA study has important implications for the risk of perioperative major adverse cardiac events (MACE) in COVID-19 survivors. In comparing more than 150k COVID-19 survivors with more than 11 million controls, the risk of post-acute cardiovascular manifestations was significantly higher in patients who had an ICU admission or were hospitalized than for those who were not hospitalized. These factors should be taken into consideration in the preoperative cardiovascular workup of COVID-19 survivors subsequently presenting for moderate or high-risk surgery. - Coronavirus Disease 2019 Temperature Trajectories Correlate With Hyperinflammatory and Hypercoagulable Subphenotypes. 1/31/22. Bhavani SV. Crit Care Med.
This is a fascinating study of 5,903 COVID-19 patients classified by a validated group-based trajectory model using oral temperatures measured during 72 hours following hospital admission and dividing the patients into 4 distinct subphenotypes: hyperthermic slow (25%) and fast (25%) resolvers; normothermic (36%) and hypothermic (15%). Clinical characteristics, biomarkers, and outcomes were compared between subphenotypes. Hypothermics had abnormal coagulation markers, suggesting a hypercoagulable subphenotype. Hyperthermic slow resolvers had elevated inflammatory markers and the highest odds of mortality, suggesting a hyperinflammatory subphenotype at high risk for respiratory failure, shock, and mortality. Authors suggest future work should investigate whether temperature trajectory subphenotypes have differential responses to treatment. Excellent figures and discussion complement the manuscript. - Risk factors and abnormal cerebrospinal fluid associate with cognitive symptoms after mild COVID-19. 1/19/22. Apple AC. Annals of Clinical Trans Neuro.
US investigators studied cognitive postacute sequelae of SARS-CoV-2 (PASC) after mild COVID in patients who reported (n=22) and did not report (n=10) symptoms using structured interviews, neuropsychological testing, and optional cerebrospinal fluid (CSF) evaluations (53%). Those with cognitive symptoms frequently had delayed onset (43%), a higher number of pre-existing cognitive risk factors (2.5 vs. 0; p=0.03) and abnormal CSF findings (77% vs. 0%; p=0.01) versus controls. Cognitive risk factors and immunologic mechanisms may contribute to cognitive PASC pathogenesis. All participants were enrolled in the Long-term Impact of Infection with Novel Coronavirus (LIINC) study (NCT04362150). Further study is recommended.
SAB Comment: Although a very small study, the findings may point toward areas for future investigation. Abnormal oligoclonal banding patterns were identified in 69% (9/13) of CSF samples from participants with cognitive PASC compared to 0% of cognitive controls (p=0.03). Objective testing of cognitive function by HAND criteria correlated poorly with symptoms, raising many questions regarding subjective vs. objective criteria for cognitive function. - Prevalence and Risk Factors of Neurologic Manifestations in Hospitalized Children Diagnosed with Acute SARS-CoV-2 or MIS-C. 12/28/21. Fink EL. Pediatric Neurology.
An established consortium found that of the 1,278 SARS-CoV-2 hospitalized children internationally, 40% suffered neurologic manifestations. Findings in this data dense study concluded that headaches were the most common symptom, yet encephalopathy and seizures were significant. Pre-existing neurologic illness increased the risk 3.48-fold. Metabolic illness also increased risk. Delirium and psychosis were rare. Most children were discharged home; death occurred in 1% of the children. - COVID-19 Incidence and Death Rates Among Unvaccinated and Fully Vaccinated Adults with and Without Booster Doses During Periods of Delta and Omicron Variant Emergence – 25 U.S. Jurisdictions, April 4-December 25, 2021. 1/27/22. Johnson AG. Morb Mortal Wkly Rep.
In an effort to determine the effectiveness of vaccination and booster shots during the Delta and Omicron surges, the CDC analyzed data from nearly 7 million COVID-19 cases from April through December 2021. Decreases in case incidence rate ratios for unvaccinated vs. fully vaccinated persons with and without booster vaccine doses were observed when the Omicron variant emerged in December 2021. Protection against infection and death during the Delta-predominant period and against infection during Omicron emergence were higher among booster vaccine dose recipients, especially among persons aged 50 and older. COVID-19 vaccination protected against SARS-CoV-2 infection, even as the Omicron variant became prominent.
SAB Comment: This statistically intricate paper is well summarized by the two graphs of cases and deaths, clearly showing the value of vaccination and boosters, including during the Omicron surge. - Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2. 1/11/22. Liu L. Nature.
The US and Hong Kong authors of this paper conclude that “the Omicron variant presents a serious threat to many existing COVID-19 vaccines and therapies, compelling the development of new interventions that anticipate the evolutionary trajectory of SARS-CoV-2”. They present data on the severely reduced neutralizing capacity of 17 of the 19 monoclonal antibodies tested and sera from convalescent patients from first wave cases (n=10), and fully vaccinated and boosted individuals following Pfizer (n=13), Moderna (n=12), Johnson & Johnson (n=9), and AstraZeneca (n=5) vaccines. Data and discussion regarding specific B.1.1.529 (Omicron) mutations are included in this short report. - Safety and immunogenicity of a high-dose quadrivalent influenza vaccine administered concomitantly with a third dose of the mRNA-1273 SARS-CoV-2 vaccine in adults aged ≥65 years: a phase 2, randomised, open-label study. 2/1/22. Izikson R. Lancet Respir Med.
These are interim results of an ongoing phase-2 industry-supported trial looking for vaccine reactogenicity in adults older than 65 years up to 21 days after receiving a Moderna mRNA-1273 booster alone, a high-dose quadrivalent flu shot alone, or coadministration of both (around 100 patients each). All had completed a 2-dose mRNA vaccination scheduled at least 5 months previously. Reactiogenicity profiles were similar between the coadministration and mRNA-1273 alone groups, with lower reactogenicity rates after the flu shot alone. No safety issues or immune interference were observed. - Additional articles and resources selected for the IARS COVID-19 Resource website and searchable by topic:
- Association of Convalescent Plasma Treatment With Clinical Status in Patients Hospitalized With COVID-19: A Meta-analysis. 1/25/22. Troxel AB. JAMA Netw Open.
- SAB Comment: The methodology used in this article was somewhat unique, using real-time individual patient data pooling from all 8 studies.
- Corticosteroids as risk factor for COVID-19-associated pulmonary aspergillosis in intensive care patients. 1/29/22. Leistner R. Crit Care.
- Neutralization of SARS-CoV-2 Omicron by BNT162b2 mRNA vaccine-elicited human sera. 1/18/22. Muik A. Science.
- Anxiety, worry, and job satisfaction: effects of COVID-19 care on critical care anesthesiologists. 1/13/22. Siddiqui S. Can J Anaesth.
- The Impact of the COVID-19 Pandemic on Mental Health, Occupational Functioning, and Professional Retention Among Health Care Workers and First Responders. 12/16/21. Hendrickson RC. Journal of General Intern Med.
- Mental health symptoms in family members of COVID-19 ICU survivors 3 and 12 months after ICU admission: a multicentre prospective cohort study. 2/1/22. Heesakkers H. Intensive Care Med.
- Challenges for the Beleaguered Health Care Workforce During COVID-19. 1/27/22. Cutler DM. JAMA Forum.
- The emergence of COVID-19 associated mucormycosis: a review of cases from 18 countries. 1/31/22. Hoenigl M. Lancet Microbe.
- Association of Convalescent Plasma Treatment With Clinical Status in Patients Hospitalized With COVID-19: A Meta-analysis. 1/25/22. Troxel AB. JAMA Netw Open.
Newsletter Issue 118, February 7, 2022:
- Multiple Early Factors Anticipate Post-Acute COVID-19 Sequelae. 1/19/22. Su Y. Cell.
These numerous authors present an extensively detailed, longitudinal profiling of 209 COVID-19 patients representing a broad spectrum of acute infection severities along with 457 healthy controls. Patients were studied at clinical diagnosis, during acute disease, and 2-3 months after onset of initial symptoms. Four features present at diagnosis (pre-existing type 2 diabetes, assessments of SARS-CoV-2 RNAemia, EBV viremia, and autoantibodies) predicted the likelihood of Post-Acute Sequelae of COVID-19 (PASC), a syndrome defined by the CDC as a range of new, returning, or ongoing health problems that people can experience four or more weeks following initial SARS-CoV-2 infection (called “Long COVID” by some). The authors note that each of the features associated with PASC in the study can be treated.
SAB Comment: This study demonstrates a statistical association between the 4 features noted and the persistence of symptoms 2-3 months later, but the authors don’t claim to show a causal relationship or predict a response to treatment. They don’t attempt to distinguish these associations from those related to intensive care hospitalization or predict the persistence of the symptoms longer than 2-3 months. - Cardiac Dysfunction and Arrhythmias 3 Months After Hospitalization for COVID-19. 1/20/22. Ingul CB. J Am Heart Assoc.
In this data-rich Norwegian study of 204 hospitalized COVID-19 survivors and 204 matched controls the authors conclude: “At 3 months after COVID‐19 hospitalization, patients had mildly impaired RV (right ventricular) function, reduced diastolic function, and mainly preserved left ventricular function compared with matched controls. Premature ventricular contractions and nonsustained ventricular tachycardia were common, but the relation to COVID‐19 is unknown. Patients treated in an ICU had similar cardiac function as those not admitted to an ICU. Persistent dyspnea or fatigue were not found to be associated with cardiac function.”
Newsletter Issue 117, January 31, 2022:
- The IARS COVID-19 Scientific Advisory Board (SAB) continually screens newly published peer-reviewed articles from respected journals to identify those of greatest clinical and scientific relevance to anesthesiologists, intensivists, related specialists and investigators. Our open-access newsletter provides a link to each highlighted article along with a short summary of key points. The SAB does not include any information from news media, social media, or scientific articles lacking full peer-review such as pre-prints.
- NIH Therapeutics Recommendations (Updated January 19, 2022)
Based upon data generated during the Omicron variant surge, the NIH has revised its recommendations for antiviral therapeutics for nonhospitalized patients with mild-moderate COVID-19 at high risk of progression to severe disease. The drugs are ranked by order of preference. For a full list, visit https://www.covid19treatmentguidelines.nih.gov/therapies/statement-on-therapies-for-high-risk-nonhospitalized-patients/.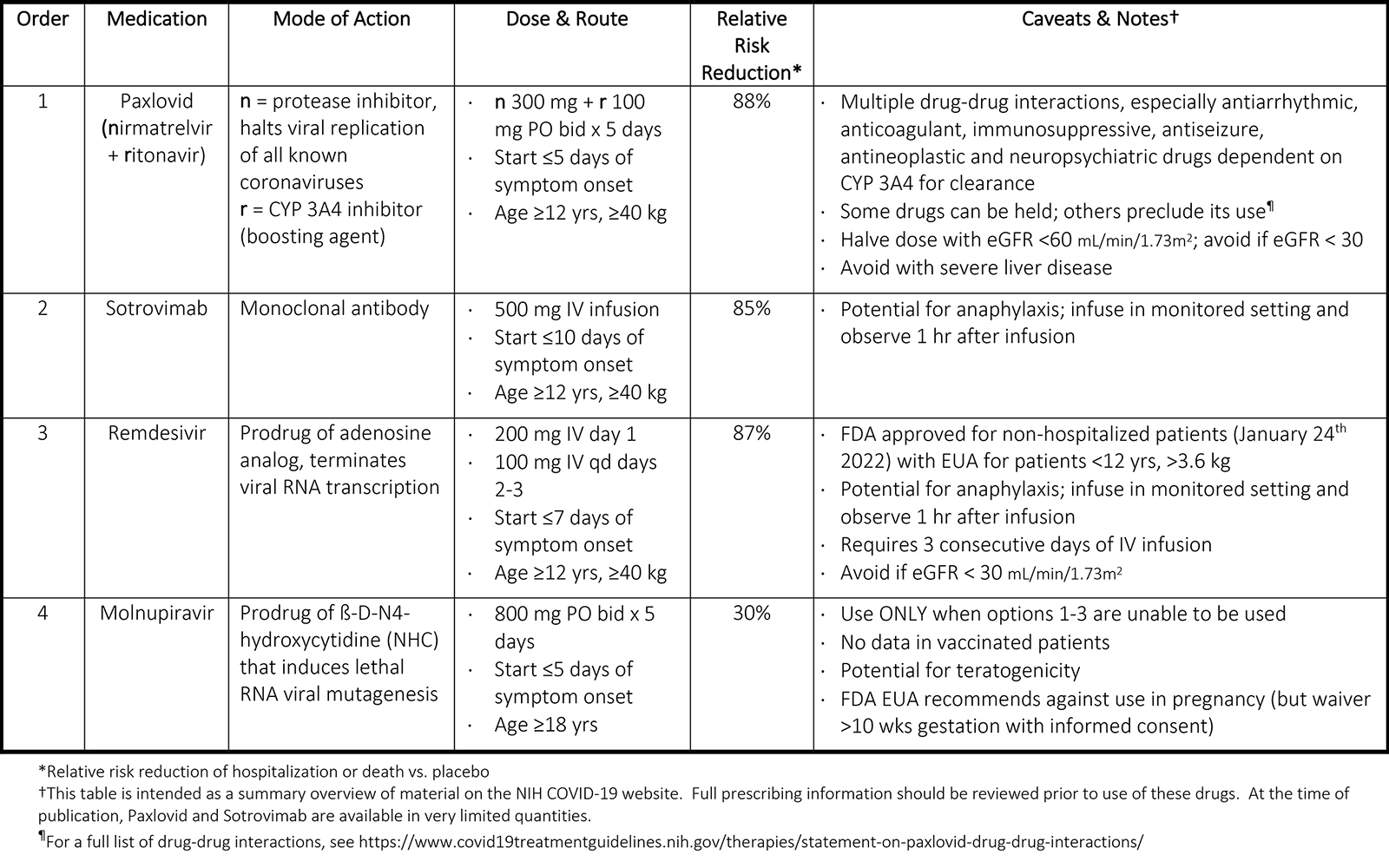
- Cannabidiol inhibits SARS-CoV-2 replication through induction of the host ER stress and innate immune responses. 1/20/22. Nguyen L. Science Advances.
The authors report that cannabidiol (CBD) and its metabolite 7-OH-CBD, but not THC or other congeneric cannabinoids tested, potently block SARS-CoV-2 replication in in-vitro human lung epithelial cells and in mouse nasal turbinates and lung tissue. In matched groups of human patients from the National COVID Cohort Collaborative, CBD treatment for seizures (100 mg/ml oral solution) had a significant negative association with positive SARS-CoV-2 tests. This study highlights CBD as a potential preventative agent for early-stage SARS-CoV-2 infection and merits future clinical trials. The authors caution against use of nonmedical formulations including edibles, inhalants or topicals as a preventative or treatment therapy at the present time. - Prone Position in Coronavirus Disease 2019 and Noncoronavirus Disease 2019 Acute Respiratory Distress Syndrome: An International Multicenter Observational Comparative Study. 9/24/21. Camporota L. Critical Care Medicine.
This is a retrospective observational multicenter study from seven ICUs in Italy, the UK and France that compared the response to prone positioning between patients with COVID-19 ARDS (n = 220) and non-COVID-19 ARDS (n = 156). Immediately before proning COVID-19 ARDS patients were more hypoxemic but had greater lung compliance than non-COVID-19 ARDS. A positive response to proning, defined by an increase ≥ 20 mmHg in PaO2/FiO2, occurred in nearly 80% of both groups and was greater the lower the initial PaO2/FiO2, and the earlier proning was commenced after intubation. Overall ICU mortality was 45%, with no significant difference between groups, but improved survival was independently associated with younger age, earlier proning and the oxygenation response to the intervention.
SAB Comment: Although this is a retrospective observational study, its strength is that it does not (again) attempt to establish whether proning is better than non-proning. Instead it explores the difference in response to proning between COVID-19 and non-COVID-19 ARDS patients, and finds that they are equivalent and that early intervention is beneficial in both groups. The COVID-19 patients were from the early pandemic (February-May 2020) so the study does not take into account the potential mortality benefit of steroid therapy that became evidence-based later in 2020. - Additional articles and resources selected for the IARS COVID-19 Resource website and searchable by topic:
- Multiple Early Factors Anticipate Post-Acute COVID-19 Sequelae. 1/19/22. Su Y. Cell.
- One-Year Multidisciplinary Follow-Up of Patients With COVID-19 Requiring Invasive Mechanical Ventilation. 1/2/22. Zangrillo A. J Cardiothorac Vasc Anesth.
- Cardiac Dysfunction and Arrhythmias 3 Months After Hospitalization for COVID-19. 1/20/22. Ingul CB. J Am Heart Assoc.
- SARS-CoV-2 breakthrough infections elicit potent, broad and durable neutralizing antibody responses. 1/20/22. Walls A. Cell.
Newsletter Issue 116, January 24, 2022:
- Perioperative Cardiovascular Considerations Prior to Elective Noncardiac Surgery in Patients With a History of COVID-19. 1/12/22. Rohatgi N. JAMA Surg.
Risk evaluation for patients with preoperative cardiac risk factors for noncardiac surgery is standard anesthesiology practice. COVID-19 is associated with cardiovascular complications including but not limited to myocardial injury, dysrhythmias, and thromboembolism. Following symptomatic recovery, optimal timing for elective surgery is discussed with data supporting a seven-week delay. Standard clinical practice guidelines can be applied to COVID-19 patients with clinical appreciation for disease severity in individual cases. A valuable table is included for reference.
SAB Comment: This opinion piece is timely given the current impact of the Omicron surge, high hospital occupancy and healthcare staff shortages on elective noncardiac surgery. However, its value may lie in stimulating discussion rather than providing a strict guideline. We have insufficient data on postacute cardiovascular manifestations and long COVID after Omicron. In a real-world situation, optimal timing of elective noncardiac surgery may depend on the interaction of institutional constraints, appropriate cardiovascular assessment and the relative “electiveness” of surgery, rather than a fixed delay. - Myocarditis and Pericarditis After Vaccination for COVID-19. 8/4/21. Diaz GA. JAMA.
This is a report on postvaccination myocarditis or pericarditis derived from data from 40 hospitals in the Providence healthcare system based in 4 states (WA, OR, MT and CA). Out of more than 2 million individuals receiving at least one vaccination (97% Pfizer or Moderna), there were 20 cases of myocarditis and 37 of pericarditis, a rate of 1.0 and 1.8 per 100k vaccinations, respectively. These represented two distinct self-limited syndromes. Myocarditis patients were mostly young (median age 36 years), male, developing symptoms 3-10 days after the second vaccination. Nineteen of 20 were admitted to hospital and all were discharged after a median of 2 days. All patients had symptom resolution or improvement within 5-40 days of onset. Pericarditis patients were also mostly male, but older (median age 59 years) with more comorbidity, developing symptoms 6-41 days after the first or second vaccination. About half (19/37) required hospital admission with a median stay of 1 day. A minority of both groups required cardiovascular therapy in addition to anti-inflammatory medications.
SAB Comment: This US-based report emphasizes that myocarditis and pericarditis are extremely rare, distinct entities occurring in different age groups, that are self-limited with a benign outcome. This risk should be compared with the risk of developing cardiovascular complications in unvaccinated patients with severe COVID-19. - Antiplatelet Therapy in Patients With COVID-19-More Is Less? 1/18/22. Spaetgens B. JAMA.
This editorial reviews a multinational prospective trial that randomized 562 non-ICU selected patients with COVID-19 to 1) therapeutic heparin or 2) therapeutic heparin with a P2Y12 inhibitor (ticagrelor 63%, clopidogrel 37%). The number of days free of respiratory or cardiovascular organ support, incidence of death in the hospital, and bleeding complications were the same between the two groups.
These data suggest no additional benefits with the use of P2Y12 inhibitors in the face of full therapeutic anticoagulation for non-ICU patients with COVID-19. These data are consistent with prior data failing to demonstrate benefit of the use of aspirin in COVID-19 patients.
SAB Comment: The trial started on February 26, 2021 but was discontinued due to futility 4 months later. - Early Remdesivir to Prevent Progression to Severe Covid-19 in Outpatients. 12/22/21. Gottlieb RL. N Engl J Med.
In this randomized, double-blinded, placebo controlled trial of 3 doses of Remdesivir (N=279 vs. placebo N=283), COVID-19 patients, ages 50+, with symptoms and one co-morbidity were studied. Remdesivir had a 87% lower risk of COVID-19–related hospitalization or death and 81% lower risk of medical visits compared to the placebo at day 28. The drug is considered to be safe in the outpatient setting. This group included patients that were unvaccinated and not on oxygen before the emergence of the Delta variant. - Debulking SARS-CoV-2 in saliva using angiotensin converting enzyme 2 in chewing gum to decrease oral virus transmission and infection. 11/10/21. Daniell H. Molecular Therapy.
This study describes the development of a chewing gum containing virus-trapping proteins expressly designed to minimize oral virus transmission or reinfection. Salivary glands are primary sites of SARS-CoV-2 replication and saliva from symptomatic or asymptomatic COVID-19 patients contains a viral load proportionate to the severity of symptoms. Contagious airborne droplets are the major form of transmission of the virus, which utilizes ACE2 receptors to enter cells. The authors incorporated biomass plant-created cholera toxin B(CTB)-ACE2 powder into flavored chewing gum and tested its ability in vitro to bind to viral spike proteins, debulk virus from saliva and thus potentially reduce oral transmission. Incubation of CTB-ACE2 microparticles with infected saliva reduced SARS-CoV-2 virus count by >95%. Since the mechanism utilizes the ACE2 receptor rather than blocking specific viral antigens, it has the potential to be effective regardless of the variant genome.
SAB Comment: This is a remarkably novel approach to the amelioration of viral transmission that if proven to be beneficial in vivo could have dramatic effects on COVID-19 contagion. It also creates the potential of allowing millions to chew gum and walk at the same time. - Ancestral SARS-CoV-2-specific T cells cross-recognize the Omicron variant. 1/18/22. Gao Y. Nat Med.
In some cases, Omicron, despite escaping antibodies, does not lead to severe disease. These investigators examined Omicron spike-specific CD4+ and CD8+ T cell responses induced by prior infection or BNT162b2 (Pfizer) vaccination. Spike-specific CD4+ T cells that cross-recognized Omicron in previously infected or BNT162b2-vaccinated individuals were 84% and 91%, respectively, and for CD8+ T cells were 70% and 92%. The CD4+ and CD8+ T cells were functionally and phenotypically similar in response to the ancestral strain or Omicron. These data indicate that established SARS-CoV-2 spike-specific CD4+ and CD8+ T cell responses, especially after BNT162b2 vaccination, remain largely intact against Omicron. - Additional articles and resources selected for the IARS COVID-19 Resource website and searchable by topic:
Newsletter Issue 115, January 19, 2022:
- Real-World Effectiveness Of Remdesivir In Adults Hospitalized With Covid-19: A Retrospective, Multicenter Comparative Effectiveness Study. 12/15/21. Garibaldi BT. Clin Infect Dis.
This is a large population study (98,000 patients, 160 hospitals across 21 states), examining the still disputed clinical effectiveness of remdesivir. Compared to matched controls, remdesivir was associated with a statistically significant increase in the likelihood of clinical improvement and survival beyond 28 days in patients on no or low-flow oxygen. Of 36,656 matched individuals, 74.0% who received remdesivir and 68.3% of controls achieved clinical improvement before 28 days with a median time to clinical improvement of 7 days (IQR 5,19) in the remdesivir recipients and 9 days (IQR 5,28) for controls. Results are consistent with the outcome of the ACTT-1 trial and underscore the role of remdesivir in patients with mild respiratory symptoms and its failure to improve advanced disease. - Rivaroxaban versus no anticoagulation for post-discharge thromboprophylaxis after hospitalisation for COVID-19 (MICHELLE): an open-label, multicentre, randomised, controlled trial. 12/18/21. Ramacciotti E. Lancet.
This trial from Brazil randomized 320 patients to receive post-hospital discharge prophylactic rivaroxaban or no anticoagulation (from Oct 8, 2020, to June 29, 2021). 165 patients (52%) had been in intensive care. Patients underwent bilateral lower-limb venous ultrasound and CT pulmonary angiogram at day 35. Rivaroxaban was associated with decreased rates of 1) symptomatic and fatal VTE 0.6% vs. 5% and 2) composite of symptomatic VTE, myocardial infarction, stroke, and cardiovascular death (0.6% vs. 5.6%). Furthermore, the magnitude of events was also decreased (more events were asymptomatic in the anticoagulant group than in the control group). This is the first trial demonstrating benefit of post-discharge prophylaxis. - Additional articles and resources selected for the IARS COVID-19 Resource website and searchable by topic:
- Omicron Variant Patient Profile in South Africa. 12/6/21. SAMRC.
Readers interested in following the progress of the Omicron variant in South Africa, where it was first identified on November 24, 2021, are directed to the website of the South African Medical Research Council (SAMRC). This site provides access to a developing patient profile of the impact of the Omicron surge on hospitalization and outcomes here. - Infectious Diseases Society of America (IDSA). 11/18/21.
This website provides Infectious Diseases Society of America (IDSA) Guidelines on the Treatment and Management of patients with COVID-19. The IDSA frequently updates the site with their carefully considered and well-organized distillation of current knowledge. In particular, their Summary Table titled, “Overview of COVID-19 Treatment Guidelines” is particularly concise and informative. This IDSA guideline link will be placed in the IARS Published Guidelines and Reviews web page for future reference.
- Omicron Variant Patient Profile in South Africa. 12/6/21. SAMRC.
Newsletter Issue 114, January 10, 2022:
- Decreased severity of disease during the first global omicron variant covid-19 outbreak in a large hospital in tshwane, south africa. 12/31/21. Abdullah F. Int J Infect Dis.
This retrospective cohort study compared 466 SARS-CoV-2 PCR+ inpatients since 11/14/21 to 3976 PCR+ inpatients during pre-Omicron waves. Deaths and ICU admissions were 4.5% vs. 21.3% (p<0.00001), and 1% vs. 4.3% (p<0.00001); length of stay was 4 vs. ~9 days; and mean age was 39 years during Omicron wave vs. 49 years for previous waves. Admissions peaked and declined rapidly with peak bed occupancy at 51% of highest previous peak. Only 1/3 of patients had COVID-19 pneumonia (45% on oxygen vs. 99% previously). Nearly 2/3 of positive PCR results were incidental to admission for other illnesses. - Characteristics and Outcomes of Hospitalized Patients in South Africa During the COVID-19 Omicron Wave Compared With Previous Waves. 12/30/21. Maslo C. JAMA Netw Open.
This report is from the South African Netcare health system representing 49 acute care hospitals. The authors compared outcomes of 17,200 patients evaluated across four COVID-19 waves (ancestral, Beta, Delta and Omicron variants). Compared with the previous waves, the Omicron wave demonstrated a decreased incidence of hospital admission (41.3 v 68-69%) despite only 24.2% of patients known to be vaccinated. Patients were younger (median 36 years v 59 years with Delta), more likely to be female and less likely to have comorbidities. Compared to the Delta wave, the proportion of admitted patients presenting with an acute respiratory condition (31.6 v 91.2%), requiring oxygen therapy (17.6 v 74%), ICU admission (18.5 v 29.9%) and mechanical ventilation (1.6 v 12.4%) was significantly less during the Omicron than the Delta wave. Median length of stay (3 v 9 days) and hospital mortality (2.7 v 29.1%) were also significantly lower. The authors acknowledge the absence of genotyping (but 95% of variants were Omicron at the time of study) and varying public health restrictions during different waves.
SAB Comment: This is one of a number of databases, and one of the very first peer-reviewed publications, suggesting that the Omicron wave is associated with less severe illness, hospitalization and death. At present it is not clear whether this difference results from enhanced acquired or natural population immunity or innately lower Omicron variant pathogenicity. It is also important to recognize that surge patterns in one country are not directly applicable to another. - An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by therapeutic monoclonal antibodies. 1/4/22. VanBlargan L. Res Sq.
This invitro study compares the ability of existing monoclonal antibodies to SARS-CoV-2 (mAb) to neutralize B1.1.529 (Omicron) versus a COVID-19 variant previously studied by the authors and known to be susceptible to mAb. Structural models were used to compare the Omicron spike to the various mAb structures. Using Vero cells, the focus reduction neutralization test demonstrated that the Regeneron, Lilly, and Celltrion mAbs completely lost the ability to neutralize Omicron. The effectiveness of AstraZeneca monoclonal antibodies was reduced 12 fold. S309 (the parent of sotrovimab) was affected least, but was less potent with cells expressing hACE2. - SAB Comment: The IARS COVID-19 SAB recommends to readers the following group of opinion articles recently published in JAMA written by several members of a pandemic scientific advisory board that counseled President Biden during his transition. We provide these links not as an IARS SAB endorsement of the views expressed, but to encourage readers to review this reflective and forward-looking discussion at the two-year mark of the COVID-19 pandemic and during the current Omicron variant surge.
- The Pandemic Preparedness Program: Reimagining Public Health. 1/6/22. Adashi EY. JAMA.
- A National Strategy for COVID-19: Testing, Surveillance, and Mitigation Strategies. 1/6/22. Michaels D. JAMA.
- A National Strategy for COVID-19 Medical Countermeasures: Vaccines and Therapeutics. 1/6/22. Borio LL. JAMA.
- A National Strategy for the “New Normal” of Life With COVID. 1/6/22. Emanuel EJ. JAMA.
- The First 2 Years of COVID-19: Lessons to Improve Preparedness for the Next Pandemic. 1/6/22. Nuzzo JB. JAMA.
- Additional articles and resources selected for the IARS COVID-19 Resource website and searchable by topic:
- Omicron Variant Patient Profile in South Africa. 12/6/21. SAMRC.
Readers interested in following the progress of the Omicron variant in South Africa, where it was first identified on November 24, 2021, are directed to the website of the South African Medical Research Council (SAMRC). This site provides access to a developing patient profile of the impact of the Omicron surge on hospitalization and outcomes here. - Infectious Diseases Society of America (IDSA). 11/18/21.
This website provides Infectious Diseases Society of America (IDSA) Guidelines on the Treatment and Management of patients with COVID-19. The IDSA frequently updates the site with their carefully considered and well-organized distillation of current knowledge. In particular, their Summary Table titled, “Overview of COVID-19 Treatment Guidelines” is particularly concise and informative. This IDSA guideline link will be placed in the IARS Published Guidelines and Reviews web page for future reference. - Reduced neutralisation of SARS-CoV-2 omicron B.1.1.529 variant by post-immunisation serum. 12/23/21. Dejnirattisai W. Lancet.
- Neutralization of SARS-CoV-2 Omicron variant by sera from BNT162b2 or Coronavac vaccine recipients. 12/16/21. Lu L. Clin Infect Dis.
- Immunogenicity, safety, and reactogenicity of heterologous COVID-19 primary vaccination incorporating mRNA, viral-vector, and protein-adjuvant vaccines in the UK (Com-COV2): a single-blind, randomised, phase 2, non-inferiority trial. 12/9/21. Stuart ASV. Lancet.
- Efficacy and Safety of NVX-CoV2373 in Adults in the United States and Mexico. 12/15/21. Dunkle LM. N Engl J Med.
- Post-ICU Syndrome in a Cohort of COVID-19 Survivors in New York City. 12/22/21. Weidman K. Ann Am Thorac Soc.
- Rivaroxaban versus no anticoagulation for post-discharge thromboprophylaxis after hospitalisation for COVID-19 (MICHELLE): an open-label, multicentre, randomised, controlled trial. 12/18/21. Ramacciotti E. Lancet.
- Real-World Effectiveness Of Remdesivir In Adults Hospitalized With Covid-19: A Retrospective, Multicenter Comparative Effectiveness Study. 12/15/21. Garibaldi BT. Clin Infect Dis.
- Omicron Variant Patient Profile in South Africa. 12/6/21. SAMRC.
International Anesthesia Research Society
