Following declarations from the US Department of Health and Human Services and World Health Organization ending the COVID-19 Public Health Emergency, the IARS COVID-19 Scientific Advisory Board (SAB) concluded its review of the scientific literature about SARS-CoV-2 in August. The SAB has reviewed more than 3,100 journal articles and published 1,076 article reviews over the past 42 months. It has been an enormous commitment from the SAB, and the IARS owes our dedicated physician volunteers a huge debt of gratitude for their unwavering participation in this initiative.
The IARS COVID-19 Scientific Advisory Board (SAB) has screened newly published peer-reviewed articles from respected journals to identify those of greatest clinical and scientific relevance to anesthesiologists, intensivists, related specialists and investigators. Our open-access newsletter provides a link to each highlighted article along with a short summary of key points. The SAB does not include any information from news media, social media, or scientific articles lacking full peer-review such as pre-prints.
To search by keyword, select Ctrl + F on a PC and Command + F on a Mac. Then, enter keyword and Enter.
The material on this website is provided for informational purposes and does not constitute medical advice. New knowledge is added daily and may change over time. Opinions expressed should not be construed as representing IARS policy or recommendations. References and links to third parties do not constitute an endorsement or warranty by IARS.
Retractions:
- Effectiveness of Surgical and Cotton Masks in Blocking SARS–CoV-2: A Controlled Comparison in 4 Patients – published on April 6, 2020 in the Annals of Internal Medicine, retracted on June 1, 2020.
- Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis – published in The Lancet on May 22, 2020, subjected to an expression of concern on June 2, and retracted on June 4.
- Cardiovascular Disease, Drug Therapy, and Mortality in Covid-19 – published in the New England Journal of Medicine on May 1, 2020, subjected to an expression of concern on June 2, and retracted on June 4.
Newsletter Issue 113, December 20, 2021:
- Effect of High-Flow Oxygen Therapy vs Conventional Oxygen Therapy on Invasive Mechanical Ventilation and Clinical Recovery in Patients With Severe COVID-19: A Randomized Clinical Trial. 12/7/21. Ospina-Tascón GA. JAMA.
This prospective, randomized, controlled, open-label trial from 3 hospitals in Colombia compared the 28-day need for intubation and the time to clinical recovery in 99 severely ill COVID-19 pneumonia patients treated with high-flow nasal cannula to those outcomes in 100 similar patients treated with standard modalities of nasal cannula and mask oxygen. Intubation was necessary in 34% of the patients randomized to high-flow oxygen therapy and in 51% randomized to conventional oxygen therapy. The median time to clinical recovery was 11 days in patients randomized to high-flow oxygen therapy vs 14 days in those randomized to conventional oxygen therapy. - Bleeding Complications in Patients With Perioperative COVID-19 Infection Undergoing Cardiac Surgery: A Single-Center Matched Case-Control Study. 12/15/21. Chiariello GA. J Cardiothorac Vasc Anesth.
The purpose of this study was to investigate a possible significant correlation between perioperative COVID-19 infection and hemorrhagic complications compared to non-COVID-19 patients who underwent on-pump, open-heart cardiac surgery from February 2020 to March 2021. Among them, 23 consecutive patients had perioperative diagnoses of COVID-19 infection (study group). These patients were compared with 46 corresponding matched controls (control group). The study group demonstrated markedly higher bleeding complications in the short and long term. In the study group, patients showed a significantly higher incidence of bleeding complications (48% v 2%, p = 0.0001) and cases of surgical reexploration for bleeding (35% v 2%, p = 0.0001), a higher incidence of severe postoperative thrombocytopenia (39% v 6%, p = 0.0007), and a higher need of blood components transfusions (74% v 30%, p = 0.0006). Chest tubes blood loss and surgical hemostasis time were markedly prolonged (p = 0.02 and p = 0.003, respectively).
SAB Comment: These data have implications for CT surgeons, CT anesthesia and intensivists but may also possibly be worth noting for other surgical teams. - Disorders of Consciousness Associated With COVID-19: A Prospective, Multimodal Study of Recovery and Brain Connectivity. 12/4/21. Fischer D. Neurology.
Disorders of consciousness (DoC) in severe COVID-19 disease carry a serious if not ominous prognosis. This prospective, longitudinal, multimodal study screened 1,105 consecutive patients admitted to MGH/Brigham’s critical care units during a 9-month period ending in March 2021 to clarify short and long-term outcomes of DoC unexplained by sedation. Only 12 patients satisfied study criteria which included scheduled advanced MRI studies to assess functional and structural brain injury. All 8 survivors regained consciousness, 50% within a week after sedation and 75% regained normal cognition and had minimal disability at 6 months. Eighty-two percent of patients demonstrated microhemorrhages and/or leukoencephalopathy on imaging, findings which did not correlate with severity of DoC and point towards a multifactorial etiology that includes, sedation, hypoxia and inflammation. Despite obvious limitations, this study adds a measure of optimism to an arena of tragic outcomes. - Additional articles and resources selected for the IARS COVID-19 Resource website and searchable by topic:
- Omicron Variant Patient Profile in South Africa. 12/6/21. SAMRC.
Readers interested in following the progress of the Omicron variant in South Africa, where it was first identified on November 24, 2021, are directed to the website of the South African Medical Research Council (SAMRC). This site provides access to a developing patient profile of the impact of the Omicron surge on hospitalization and outcomes here. - Infectious Diseases Society of America (IDSA). 11/18/21.
This website provides Infectious Diseases Society of America (IDSA) Guidelines on the Treatment and Management of patients with COVID-19. The IDSA frequently updates the site with their carefully considered and well-organized distillation of current knowledge. In particular, their Summary Table titled, “Overview of COVID-19 Treatment Guidelines” is particularly concise and informative. This IDSA guideline link will be placed in the IARS Published Guidelines and Reviews web page for future reference. - Lenzilumab in hospitalised patients with COVID-19 pneumonia (LIVE-AIR): a phase 3, randomised, placebo-controlled trial. 12/5/21. Temesgen Z. Lancet Respir Med.
- Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. 12/15/21. Patone M. Nat Med.
- Incidence of venous thromboembolic events in COVID-19 patients after hospital discharge: A systematic review and meta-analysis. 12/13/21. Zuin M. Thromb Res.
- Omicron Variant Patient Profile in South Africa. 12/6/21. SAMRC.
The material on this website is provided for informational purposes and does not constitute medical advice. New knowledge is added daily and may change over time. Opinions expressed should not be construed as representing IARS policy or recommendations. References and links to third parties do not constitute an endorsement or warranty by IARS.
Retractions:
- Effectiveness of Surgical and Cotton Masks in Blocking SARS–CoV-2: A Controlled Comparison in 4 Patients – published on April 6, 2020 in the Annals of Internal Medicine, retracted on June 1, 2020.
- Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis – published in The Lancet on May 22, 2020, subjected to an expression of concern on June 2, and retracted on June 4.
- Cardiovascular Disease, Drug Therapy, and Mortality in Covid-19 – published in the New England Journal of Medicine on May 1, 2020, subjected to an expression of concern on June 2, and retracted on June 4.
Newsletter Issue 112, December 13, 2021:
- BNT162b2 Vaccine Booster and Mortality Due to Covid-19. 12/8/21. Arbel R. N Engl J Med.
This report comes from the records of 843,308 Clalit Health (providing 50% of Israeli healthcare) patients older than 50 years with 2 doses of BNT162b2 for at least 5 months. During the study from 8/6/2021 – 9/29/2021, as patients received a third dose, they were compared to those having only 2 doses, with a primary outcome of mortality and a secondary outcome of testing positive for SARS-CoV-2. At the end of the study, 90% of patients had received a third dose. For the primary outcome the relative risk of mortality after the third dose was 0.10, a 90% lower mortality rate. - HSV-1 reactivation is associated with an increased risk of mortality and pneumonia in critically ill COVID-19 patients. 12/7/21. Meyer A. Crit Care.
This French prospective, observational study examined Herpes simplex virus-1 (HSV-1) reactivation in 153 severe COVID-19 patients. Outcomes were mortality, hospital-acquired pneumonia, ventilator-associated pneumonia (HAP/VAP) and bloodstream infection (BSI). HSV-1 respiratory and blood samples were collected in patients admitted for 48 hours or more (Feb-2020 to Feb-2021). HSV-1 reactivation occurred in 40/153 (26.1%) of patients. Respiratory samples were positive in 19/61 (31.1%) and/or blood samples positive in 36/146 (24.7%). HSV-1 reactivation in critically ill COVID-19 patients was associated with marked increased risk of day-60 mortality (57.5% with versus 33.6% without, p=0.001) and with HAP and VAP (p=.037), but not BSI.
SAB Comment: This and other smaller studies document HSV-1 reactivation during COVID and non-COVID ARDS. To date, no study has determined worsening outcomes because of HSV-1 reactivation producing further lung injury or whether reactivation is an “epiphenomenon” resulting from disease-induced immunosuppression (e.g., lymphopenia, etc.) and not further lung injury. Also, thus far, only poorly powered studies have failed to show that initiation of antivirals improve outcomes. Much research remains in this area of viral reactivation in ARDS. - Additional articles and resources selected for the IARS COVID-19 Resource website and searchable by topic:
- Omicron Variant Patient Profile in South Africa. 12/6/21. SAMRC.
Readers interested in following the progress of the Omicron variant in South Africa, where it was first identified on November 24, 2021, are directed to the website of the South African Medical Research Council (SAMRC). This site provides access to a developing patient profile of the impact of the Omicron surge on hospitalization and outcomes here. - Infectious Diseases Society of America (IDSA). 11/18/21.
This website provides Infectious Diseases Society of America (IDSA) Guidelines on the Treatment and Management of patients with COVID-19. The IDSA frequently updates the site with their carefully considered and well-organized distillation of current knowledge. In particular, their Summary Table titled, “Overview of COVID-19 Treatment Guidelines” is particularly concise and informative. This IDSA guideline link will be placed in the IARS Published Guidelines and Reviews web page for future reference. - Odds of Testing Positive for SARS-CoV-2 Following Receipt of 3 vs 2 Doses of the BNT162b2 mRNA Vaccine. 11/30/21. Patalon T. JAMA Intern Med.
- A Higher Antibody Response Is Generated With a 6- to 7-Week (vs Standard) Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Vaccine Dosing Interval. 12/1/21. Grunau B. Clin Infect Dis.
- Efficacy of Early Treatment with Favipiravir on Disease Progression among High Risk COVID-19 Patients: A Randomized, Open-Label Clinical Trial. 12/1/21. Chuah CH. Clin Infect Dis.
- Omicron Variant Patient Profile in South Africa. 12/6/21. SAMRC.
Newsletter Issue 111, December 6, 2021:
- The removal of airborne SARS-CoV-2 and other microbial bioaerosols by air filtration on COVID-19 surge units. 10/30/21. Conway Morris A. Clin Infect Dis.
A British COVID-19 surge ward and an ICU surge unit that were ventilated with 2-4 air changes/hour at baseline were equipped with HEPA filtration + UV sterilization units that circulated 5-10 room-volumes/hour. Air sampling was done using National Institute for Occupational Safety and Health BC 251 two-stage cyclone aerosol samplers and analyzed for SARS-CoV-2 with PCR testing. On the ward, sampling revealed SARS-CoV-2 by PCR on most days during the two weeks without operation of the HEPA/UV units, however none during daily sampling during the week with the unit operating. In contrast to the ward, in the ICU limited evidence of airborne SARS-CoV-2 was found with the filter off, and only a single sample was positive with the filter on. Ten other pathogens that were detected at baseline were also nearly absent during filtration. The authors conclude that air HEPA/UV filtration and sterilization may reduce the risk of COVID-19 and other disease transmission in hospitals. - Dexamethasone in hospitalised coronavirus-19 patients not on intensive respiratory support. 11/26/21. Crothers K. Eur Respir J.
This real-world observational study from US Veterans Administration hospitals found that, in patients not requiring high levels of oxygen support, dexamethasone treatment produced no mortality benefit, and in patients on room air, dexamethasone treatment appeared to cause harm. The authors compared the 90-day mortality of patients on room air treated with dexamethasone (n=3124) to those on room air not treated with dexamethasone (n=6006) and found that, after propensity score weighting, dexamethasone-treated patients had a 76% increase in 90-day mortality. The same comparison in patients supported with oxygen by nasal cannula showed that dexamethasone treated patients (n=4383) did not have a statistically significant improvement in 90-day mortality over nasal cannula supported patients not treated with dexamethasone (n=1437).
SAB Comment: The higher mortality in patients not on oxygen treated with dexamethasone confirms guidelines issued by the NIH and others. - Aspirin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. 11/20/21. RECOVERY Collaborative Group. Lancet.
A randomized platform trial (RECOVERY) evaluating aspirin 150 mg with usual care (7351 patients), versus usual care alone (7541 patients), was carried out between 11/01/2020 and 3/21/2021 in multiple hospitals worldwide. The primary outcome was 28 day mortality, which was 17% in both groups. Hospital discharge in 28 days was 75% in the aspirin group and 74% in the control group (p=0.0062). A 0.6% reduction of thrombotic events in the aspirin group was cancelled out by an identical increase in major bleeding events.
SAB Comment: There is little information on the value of aspirin as a therapy for COVID-19. Although the authors support further study for patients who are not hospitalized, it is clearly stated this trial does not support the addition of 150 mg of aspirin to the usual treatment of hospitalized COVID-19 patients. - Additional articles and resources selected for the IARS COVID-19 Resource website and searchable by topic:
- Omicron Variant Patient Profile in South Africa. 12/6/21. SAMRC.
Readers interested in following the progress of the Omicron variant in South Africa, where it was first identified on November 24, 2021, are directed to the website of the South African Medical Research Council (SAMRC). This site provides access to a developing patient profile of the impact of the Omicron surge on hospitalization and outcomes here. - Infectious Diseases Society of America (IDSA). 11/18/21.
This website provides Infectious Diseases Society of America (IDSA) Guidelines on the Treatment and Management of patients with COVID-19. The IDSA frequently updates the site with their carefully considered and well-organized distillation of current knowledge. In particular, their Summary Table titled, “Overview of COVID-19 Treatment Guidelines” is particularly concise and informative. This IDSA guideline link will be placed in the IARS Published Guidelines and Reviews web page for future reference. - Prostacyclin in Mechanically Ventilated Patients with COVID-19 and Severe Endotheliopathy: A Multicenter, Randomized, Clinical Trial. 11/23/21. Johansson PI. Am J Respir Crit Care Med.
- Intermediate-to-therapeutic versus prophylactic anticoagulation for coagulopathy in hospitalized COVID-19 patients: a systemic review and meta-analysis. 11/25/21. Zhang S. Thromb J.
- A randomized controlled study of convalescent plasma for individuals hospitalized with COVID-19 pneumonia. 11/17/21. Bar KJ. J Clin Invest.
- Do the Selective Serotonin Reuptake Inhibitor Antidepressants Fluoxetine and Fluvoxamine Reduce Mortality Among Patients With COVID-19? 11/15/21. Hoertel N. JAMA Netw Open.
- Effect of Prone Positioning on Clinical Outcomes of Non-Intubated Subjects with COVID-19: A Comparative Systematic Review and Meta-Analysis. 11/10/21. Beran A. Respir Care.
- Omicron Variant Patient Profile in South Africa. 12/6/21. SAMRC.
Newsletter Issue 110, November 29, 2021:
- Bain H: Innovative Modification of Bain Circuit for the Resuscitation and Transportation of Patients With Coronavirus Disease 2019. 10/21/21. Jain A. A A Pract.
The author provides a practical solution for transportation and resuscitation of COVID-19 patients with an innovative modified breathing circuit to decrease healthcare worker exposure from droplets and airborne contamination. The authors termed this modified breathing circuit as the Bain H circuit, where “H” stands for high efficiency particulate air (HEPA) filter which contains exhalation contaminants and accommodates high-flow circuit requirements. The article is accompanied with helpful illustrations.
SAB Comment: The conventional Bain circuit = coaxial modification of the Mapleson D system. - Colchicine in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. 10/21/21. RECOVERY Collaborative Group. Lancet Respir Med.
Colchicine offers no benefits in treating hospitalized COVID-19 patients especially in light of current therapeutic options.
Newsletter Issue 109, November 22, 2021:
- Laboratory-Confirmed COVID-19 Among Adults Hospitalized with COVID-19–Like Illness with Infection-Induced or mRNA Vaccine-Induced SARS-CoV-2 Immunity — Nine States, January–September 2021. 10/29/21. Bozio C. MMWR Morb Mortal Wkly Rep.
This is a CDC-led study in nine US states which looked at the protective immunity produced by a COVID-19 illness compared to that of the mRNA vaccines. Among COVID-19-like illness hospitalizations among adults aged 18 years or older whose previous infection or vaccination occurred 90-179 days earlier, the adjusted odds of laboratory-confirmed COVID-19 among unvaccinated adults with previous SARS-CoV-2 infection were 5.49-fold higher than the odds among fully vaccinated recipients of an mRNA COVID-19 vaccine who had no previous documented infection. The authors conclude that all eligible persons should be vaccinated, including unvaccinated persons previously infected with SARS-CoV-2. - Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. 11/10/21. Barda N. Lancet.
This Israeli real-world study supports the effectiveness of 3rd Pfizer “booster” doses for individuals having received 2 doses at least 5 months previously. From 7/30/21-9/23/21, 738,321 individuals were matched with an equal number of controls who had received 2 doses. Enrollment excluded healthcare workers, long-term-care residents, and those medically confined to their homes. Vaccine efficacy 7-55 days following a third dose was estimated at 93% (231 events for 2 doses vs 29 events for 3) for hospital admission, 92% (157 vs 17) for severe disease, and 81% (44 vs 7) for COVID-19-related death. - Viral loads of Delta-variant SARS-CoV-2 breakthrough infections after vaccination and booster with BNT162b2. 11/3/21. Levine-Tiefenbrun M. Nat Med.
To determine whether diminished effectiveness of the Pfizer BioNTech vaccine in reducing viral loads is due to a predominance of the Delta variant or related to loss of potency over time, the authors retrospectively analyzed quantitative PCR tests taken at an Israeli healthcare system during their Delta surge in July and August 2021, from 13,000 fully vaccinated adults, 3,000 unvaccinated adults and 500 adults who had received booster shots. Viral load in PCR-positive individuals was estimated by PCR-cycle thresholds to be lower in vaccinated than unvaccinated individuals. Viral load in breakthrough infections increased steadily from 2 months to 6 months after vaccination yet was again reduced following booster shots. These results were interpreted as evidence that boosters restore protection against the Delta variant. - Evaluation of the BNT162b2 Covid-19 Vaccine in Children 5 to 11 Years of Age. 11/9/21. Walter EB. N Engl J Med.
The C4591007 Clinical Trial Group studied the Pfizer BNT162b2 vaccine in children 5-11 years old in a multi-national study and found a 90%+ effectiveness at a low dose of 10 mcg given twice IM separated by 21 days. Overall, fatigue was the most common side effect at 0.9%. Fever was present in 8.7% of the second-dose children. - SAB Comment: This article below offers good evidence and excellent tables for those seeking additional information on mRNA vaccine efficacy.
Association Between mRNA Vaccination and COVID-19 Hospitalization and Disease Severity. 11/4/21. Tenforde MW. JAMA.
This data from 21 US hospitals updates information on mRNA vaccination effectiveness for 1,983 vaccinated and unvaccinated patients hospitalized for COVID-19 from March 11, 2021-August 15, 2021. In a negative case control design, they were matched to 1,359 respiratory-symptomatic COVID-19 negative controls and 1,171 COVID-19 negative patients hospitalized for other reasons. The adjusted odds ratio for hospitalization in individuals fully vaccinated with an mRNA vaccine was 0.15. Disease progression to death or invasive mechanical ventilation by day 28 was associated with decreased likelihood of vaccination (12.0% vs 24.7%; aOR, 0.33). Odds ratios for age, ethnicity, immunocompromised, virus variant, specific vaccine and immunization date are included. - Additional new articles selected for the IARS COVID-19 Resource website and searchable by topic:
- Bain H: Innovative Modification of Bain Circuit for the Resuscitation and Transportation of Patients With Coronavirus Disease 2019. 10/21/21. Jain A. A A Pract.
- Colchicine in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. 10/21/21. RECOVERY Collaborative Group. Lancet Respir Med.
Newsletter Issue 108, November 15, 2021:
- Association Between COVID-19 Diagnosis and In-Hospital Mortality in Patients Hospitalized With ST-Segment Elevation Myocardial Infarction. 10/29/21. Saad M. JAMA.
This is an outcome study of propensity matched cohorts (80,449 patients) with STEMI pre-admission and during admission prior to and during the COVID-19 pandemic (time periods January-December 2019 and 2020 with final follow up in January 2021). Pre-admission STEMI mortality 15.2% +’ve COVID Vs. 11.2% -ve COVID(p=.007); in-hospital developed STEMI mortality 78.5% +’ve COVID Vs. 46.1% -“veCOVID (p<.001). Mortality was the highest in 50-75-year-old Hispanic men. Pre-admission STEMI appears to have similar rates of interventional treatment in both time periods but the 2020 STEMI cohort was treated with fibrinolytics with fewer invasive interventions. The accompanying editorial provides context and adds an interesting perspective. - The immunology of asymptomatic SARS-CoV-2 infection: what are the key questions? 10/20/21. Boyton RJ. Nat Rev Immunol.
This article is a well-referenced analysis of the often-contradictory data regarding SARS-CoV-2 asymptomatic infection (AI). Prevalence of AI vs. symptomatic infection (SI) vary widely from 20-80% of total cases, higher in younger populations. In Wuhan, even without symptoms, one-third had computer tomography lung changes. The immune basis of AI vs. SI remains unclear: viral and neutralizing antibody titers appear equivalent. AI viral-shedding is shorter and antibody titers decline more rapidly. AI is not “benign”; up to 19% result in “long COVID.” AI-adaptive immunity appears strong and primes immune memory. Additionally, silent viral carriage can result in future variants of concern.
Newsletter Issue 107, November 8, 2021:
- Elastomeric Respirators for COVID-19 and the Next Respiratory Virus Pandemic: Essential Design Elements. 10/19/21. Bowdle TA. Anesthesiology.
Although healthcare workers have relied primarily on disposable filtering facepiece respirators (such as N95) during the COVID-19 pandemic, reusable elastomeric respirators (typically used for industrial purposes) have potential advantages for the COVID-19 and future respiratory virus pandemics. These advantages include improved fit, better inspiratory filtration and less waste. However, currently available elastomeric respirators were not designed primarily for healthcare or pandemic use and require further development to improve their suitability for this application. This article explores the advantages of elastomeric respirators, and the adaptations needed (such as eliminating the expiratory valve) to make them useful for healthcare providers. Table 1 (Respiratory Protection Terminology) and good photos provide an excellent background for understanding respirators. - Early Treatment for Covid-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab. 10/27/21. Gupta A. N Engl J Med.
This is an interim analysis of COMET-ICE, a multicenter, double-blinded, randomized, phase 3 trial for COVID-19 outpatients with symptoms less than or equal to 5 days with an additional comorbidity, which were treated with Sotrovimab 500 mg IV (n = 291) or placebo (n = 292). An interim analysis revealed that 1% of the treated group as compared to 7% in the placebo group had disease progression leading to hospitalization or death (relative risk reduction 85%). The authors noted low adverse events and retained activity against variants of concern, including the alpha, beta, gamma, delta, and lambda variants. The drug can address the unmet needs of patients who have vaccine breakthrough, are unvaccinated, or are immunocompromised and may not respond to a vaccine.
SAB Comment: The drug is promising for COVID-19. It acts against the non-receptor-binding motif, unlike current authorized COVID-19 monoclonal antibodies, which act on the receptor-binding motif. - A randomized, placebo-controlled clinical trial of bamlanivimab and etesevimab together in high-risk ambulatory patients with COVID-19 and validation of the prognostic value of persistently high viral load. 10/31/21. Dougan M. Clin Infect Dis.
This Phase 3 portion of the industry-funded BLAZE-1 trial characterized the effect of bamlanivimab (700mg) with etesevimab (1400mg) infusion at this lower dose on overall patient clinical status and virologic outcomes in ambulatory patients 12 years or older, with mild-to-moderate COVID-19, and 1 or more risk factors for progressing to severe COVID-19 and/or hospitalization. Of 769 patients, the difference in hospitalization and/or death within 29 days for those receiving these monoclonal antibodies vs. placebo was 0.8% vs. 5.8% respectively. All 4 recorded deaths were in the placebo group. Persistently high viral load at Day 7, previously observed and considered a predictor of progression to a more serious form of COVID-19, was corroborated in this phase of the trial.
SAB Comment: In February, 2021, the FDA issued an Emergency Use Authorization (EUA) for the doses used in this study, in mild to moderate COVID-19 in adults and pediatric patients (12 years of age and older weighing at least 40 kg). In September this EUA was extended to include post-exposure prophylaxis. This EUA excludes states with resistance to these drugs over 5% (see FDA release for the most recent authorized areas). - Additional new articles selected for the IARS COVID-19 Resource website and searchable by topic:
- Association Between COVID-19 Diagnosis and In-Hospital Mortality in Patients Hospitalized With ST-Segment Elevation Myocardial Infarction. 10/29/21. Saad M. JAMA.
- Pre-admission anticoagulant therapy and mortality in hospitalized COVID-19 patients: A retrospective cohort study. 10/23/21. van Haaps TF. Thromb Res.
- Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial. 10/22/21. Reis G. Lancet Global Hlth.
- Effect of 12 mg vs 6 mg of Dexamethasone on the Number of Days Alive Without Life Support in Adults With COVID-19 and Severe Hypoxemia: The COVID STEROID 2 Randomized Trial. 10/21/21. COVID STEROID 2 Trial Group. JAMA.
- Investigating Lipid-Modulating Agents for Prevention or Treatment of COVID-19: JACC State-of-the-Art Review. 10/15/21. Talasaz AH. J Am Coll Cardiol.
- Neurological complications after first dose of COVID-19 vaccines and SARS-CoV-2 infection. 10/26/21. Patone M. Nat Med.
- Brain MRI and neuropsychological findings at long-term follow-up after COVID-19 hospitalisation: an observational cohort study. 10/28/21. Hellgren L. BMJ Open.
- Assessment of Cognitive Function in Patients After COVID-19 Infection. 10/22/21. Becker JH. JAMA Netw Open.
- The immunology of asymptomatic SARS-CoV-2 infection: what are the key questions? 10/20/21. Boyton RJ. Nat Rev Immunol.
Newsletter Issue 106, October 25, 2021:
- SAB Comment: The SAB is awaiting publication of peer-reviewed data regarding the efficacy of Merck’s oral antiviral molnupiravir prior to evaluating this potentially effective therapy for our newsletter.
- Administration of Monoclonal Antibody for COVID-19 in Patient Homes. 10/14/21. Malani AN. JAMA Netw Open.
In this research letter, during the spring 2021 COVID-19 surge with peak counts of 1,300 cases per day, a Michigan healthcare system introduced successful in-home IV administration of monoclonal antibodies by paramedics to 144 high-risk COVID-19 patients with mild symptoms. This resulted in decompression of hospital facilities with only 8 patients requiring hospital admissions and no intubations or mortalities. Triage was accomplished by 3 nurses and prime risk factors were obesity, cardiovascular disease, and diabetes. - REGEN-COV Antibody Combination and Outcomes in Outpatients with Covid-19. 9/29/21. Weinrich D. NEJM.
This report represents the phase 3 portion of Regeneron’s adaptive trial to demonstrate the efficacy of a combination of two monoclonal antibodies (imdevimab and casirivimab) in outpatients with COVID-19 and risk factors for severe disease in comparison to placebo. The phase 1-2 portion of this trial published one year ago showed a reduction of viral load and medical visits in 275 symptomatic patients. This phase compared 3 larger groups of patients, each receiving the antibody combination in two different doses or placebo. A highly significant relative risk reduction of 71% for hospitalization or death over placebo and a 4-day reduction in resolution of symptoms underlined the value of this treatment modality. In addition, the authors conclude that effectiveness does not depend on baseline serum antibody status. - Effect of Antithrombotic Therapy on Clinical Outcomes in Outpatients With Clinically Stable Symptomatic COVID-19: The ACTIV-4B Randomized Clinical Trial. 10/11/21. Connors JM. JAMA.
Dosing strategy for inpatient COVID-19 patients remains controversial and anticoagulation benefits for stable outpatients has not been established. The ACTIV-4B was designed as minimal contact, adaptive, randomized, double-blind, placebo-controlled study to compare anticoagulant and antiplatelet therapy among 7,000 symptomatic but stable outpatients without comorbidities. Random 1:1:1:1 allocation ratio was applied to aspirin 81mg QD (164); prophylactic apixaban 2.5mg BID; therapeutic apixaban 5mg BID (164); or placebo (164) for 45 days. Primary endpoint composite was for all-cause mortality, symptomatic venous or arterial thromboembolism, MI, stroke or hospital admission for CV or pulmonary cause. The trial was terminated by the monitoring board for lower event rate than predicted and no therapeutic difference was noted. - Antithrombotic Therapy for Outpatients With COVID-19: Implications for Clinical Practice and Future Research. 10/11/21. Berwanger O. JAMA.
This editorial review provides a comparison of multiple studies on antithrombotic therapy for inpatients and outpatients with specific review of the ACTIV-4B trial. The discussion provides insight into the studies and suggests the use of aspirin or apixaban for symptomatic but stable outpatients is not justified. Additional comments support the importance of appropriately structured and controlled clinical trials despite the difficulties associated with such studies during a pandemic. - Efficacy and Safety of Therapeutic-Dose Heparin vs Standard Prophylactic or Intermediate-Dose Heparins for Thromboprophylaxis in High-risk Hospitalized Patients With COVID-19: The HEP-COVID Randomized Clinical Trial. 10/7/21. Spyropoulos AC. JAMA Intern Med.
This multicenter US study of 253 hospitalized adults with COVID-19 and evidence of coagulopathy reinforces findings in previous publications. After randomizing those with a D-dimer greater than 4x the upper limit of normal or a sepsis-induced coagulopathy score of greater than 4, to (1) standard prophylactic or intermediate-dose low-molecular-weight heparin (LMWH) or unfractionated heparin or (2) therapeutic-dose LMWH throughout hospitalization, the primary efficacy outcome of venous thromboembolism, arterial thromboembolism, or all-cause mortality was significantly reduced with therapeutic-dose anticoagulation in the non–ICU patients only (29 vs. 42%). - Anticoagulant Therapy in Patients Hospitalized With COVID-19. 10/7/21. Wahid L. JAMA Intern Med.
This well-written accompanying editorial to the previous article discusses these findings along with those from several other major related studies, and points out coagulation management issues, such as antiplatelet therapy and extended prophylaxis, which remain unanswered. - Bacterial Superinfection Pneumonia in Patients Mechanically Ventilated for COVID-19 Pneumonia. 8/17/21. Pickens CO. ATS.
This Northwestern University single-center study examined the prevalence and etiology of bacterial superinfection (bacterial infection in addition to SARS-CoV-2) in severe SARS-CoV-2 pneumonia at intubation and subsequent ventilator-associated pneumonia (VAP). All patients had bronchoalveolar lavage (BAL) analyzed by quantitative cultures and multiplex PCR. In 179 patients, initial superinfection was detected in 21%; 44.4% developed 1 or more VAP episode(s). Initial VAP pathogens were usually CAP-type and not requiring broad spectrum coverage. Clinical criteria could not distinguish patients with or without superinfections. BAL-based management resulted in significantly reduced antibiotic use. Current guidelines, which advocate empirical antibiotics in severe SARS, results in antibiotic overuse at intubation. Forty-four percent VAPs suggests widespread under-recognition yet overtreatment with unnecessarily broad antibiotics.
Newsletter Issue 105, October 18, 2021:
- Platelets Contribute to Disease Severity in COVID-19. 9/19/21. Barrett TJ. J Thromb Haemost.
Platelet count and mean volume (MPV) were reviewed retrospectively in 3915 COVID-19 positive patients from March to June 2020. Immature platelet fraction (IPF) was followed in an additional 427 patients. Low platelet count, immaturity, and large size were associated with increased severity of disease and mortality. Additionally, autopsy specimens were obtained, and platelet RNA sequencing was carried out in 8 patients. Interestingly, there was evidence of replicating SARS-CoV-2 within a lung megakaryocyte. The authors found evidence that SARS-CoV-2 interacts with megakaryocytes to produce large immature hyperreactive platelets. The authors are investigating platelet-directed therapy.
SAB Comment: This strength of paper lies in the availability of platelet immature fraction and platelet volume from routine lab testing. These data highlight the role of platelets in the immunothrombosis of COVID-19 and raise excellent further questions for therapeutics. - External validation of prognostic scores for COVID-19: a multicenter cohort study of patients hospitalized in Greater Paris University Hospitals. 9/29/21. Lombardi Y. Intensive Care Med.
This retrospective study of 14,343 consecutive COVID-19 patients hospitalized in Paris facilities between 1/2020 and 4/2021 analyzed the efficacy of 32 published prognostic scores for predicting 30-day mortality, or need for ICU admission in addition to mortality. Areas under the receiver operating characteristic curves (AUC) were computed for each of the prognostic scores using the data for the Paris patients. Accuracy was defined as an AUC of 0.8. With AUCs of 0.79 for 30-day mortality, the 4C Mortality Score and the ABCS stand out. They performed as well in this cohort as in their initial validation cohort, and in secondary analyses during the first epidemic wave, subsequent waves, and in younger and older patients. A separate table summarizes data for the 7 best prognostic scores. For those clinicians needing to apply a prognostic instrument, the tables and supplemental material are a treasure trove of information. - Waning of BNT162b2 Vaccine Protection against SARS-CoV-2 Infection in Qatar. 10/6/21. Chemaitelly H. N Engl J Med.
To determine BNT152b vaccine (Pfizer) protection over time, these investigators followed all vaccinated Qatari patients from January 1 to September 5, 2021. Eighty percent of the population had received two doses of vaccine by September 7, PCR testing is robust (5% of the population is tested weekly), all PCR samples are genetically sequenced for variants, and centralized, coordinated data collection on all patients was routine. Vaccine effectiveness at one month after the second dose was maximum at 77.5%. Effectiveness against all breakthrough infections declined gradually thereafter, with the decline accelerating after the fourth month to reach approximately 20% in months 5 through 7. Variant-specific effectiveness waned in the same pattern. Effectiveness against severe, critical, or fatal COVID-19 reached 96% or higher in the first 2 months after the second dose and persisted at approximately this level for 6 months.
SAB Comment: This study from Qatar, and the similar study below from California, are the real-life documentation of waning effectiveness of the Pfizer vaccine, which had been suspected from lab studies documenting waning immune humoral response (https://www.nejm.org/doi/10.1056/NEJMoa2114583). Note that the vaccine shows continued effectiveness against severe disease, and that the effectiveness over time is similar for SARS-CoV-2 variants. - Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. 10/4/21. Tartof S. Lancet.
This US retrospective study differentiates the effect of the delta variant from waning immunity for the BNT162b2 (Pfizer) vaccine as a cause of increased breakthrough infections 6 months after full vaccination. Individuals (n = 1,043,289) had an overall vaccine effectiveness of 88% during the first month after full vaccination, which declined to 47% after 5 months. Effectiveness against hospital admission was 87% after one month of full vaccination, and 88% after 5 months. A significant difference in waning between variant types was not observed. Real-world variant-specific vaccine effectiveness suggested that reductions in Pfizer vaccine effectiveness rather than the delta variant escaping vaccine protection was the primary cause of vaccine breakthrough infections. - Tocilizumab administration for the treatment of hospitalized patients with COVID-19: A systematic review and meta-analysis. 10/4/21. Kyriakopoulos C. Respirology.
This well-written review includes 52 studies (nine randomized controlled trials [RCTs] and 43 observational) with a total of 27,004 patients. In both RCTs and observational studies, the use of tocilizumab was associated with a reduction in mortality; 11% in RCTs and 31% in observational studies. The need for mechanical ventilation was reduced by 19% in RCTs. Both RCTs and observational studies showed a benefit from tocilizumab on the composite endpoint of mortality or need for mechanical ventilation. Tocilizumab improved mortality both in ICU and non-ICU patients. - Tocilizumab and remdesivir in hospitalized patients with severe COVID-19 pneumonia: a randomized clinical trial. 10/5/21. Rosas IO. Intensive Care Med.
In this randomized controlled trial of patients with severe COVID-19 pneumonia, 434 were randomly assigned to tocilizumab plus remdesivir and 215 to placebo plus remdesivir. Of the 649 patients, 88.2% received corticosteroids. The median time from randomization to hospital discharge or “ready for discharge” was 14 days with tocilizumab plus remdesivir and 14 days with placebo plus remdesivir. Although large platform trials showed a survival benefit of tocilizumab in patients with severe COVID-19 and declining respiratory status, this trial did not confirm treatment benefit of tocilizumab in combination with remdesivir.
SAB Comment: Although the use of tocilizumab along with steroids is recommended in a number of major guidelines (WHO, NIH, IDSA), this industry-sponsored RCT fails to confirm its effectiveness when used with steroids and the antiviral, remdesivir. - Clinical Characteristics of Multisystem Inflammatory Syndrome in Adults: A Systematic Review. 9/22/21. Patel P. JAMA Netw Open.
Multisystem Inflammatory Syndrome in Adults (MIS-A) is exceedingly rare. These CDC authors identified 221 patients aged 18 and older, 122 of which were previously included in the CDC Multisystem Inflammatory Syndrome in Children (MIS-C) database which included 18–21-year-olds. In MIS-A, median age was 21; 70% male, 36% non-Hispanic Black, 58% without comorbidity, and most with a previous symptomatic COVID-19 like illness. Most present febrile and hypotensive, with half exhibiting cardiac dysfunction, dyspnea and diarrhea and 7% died. MIS-A is a serious hyperinflammatory condition that presents approximately 4 weeks after onset of COVID-19 with extrapulmonary multiorgan dysfunction.
SAB Comment: A conundrum presented by this paper is that the authors, themselves members of the CDC, changed the CDC definition of MIS-C from less than 21 years of age to less than 18 years of age. It could be argued that the division of MIS into MIS-C and MIS-A at a particular age is in fact arbitrary, and that MIS is a continuum of one pathologic process: a delayed inflammatory response to SARS-CoV-2, whose manifestations change with age under the influence of a changing immunomodulatory milieu. - Additional new articles selected for the IARS COVID-19 Resource website and searchable by topic:
- Beneficial Effect of Prone Positioning During Venovenous Extracorporeal Membrane Oxygenation for Coronavirus Disease 2019. 9/28/21. Zaaqoq AM. Crit Care Med.
- Extracorporeal membrane oxygenation for COVID-19: evolving outcomes from the international Extracorporeal Life Support Organization Registry. 10/2/21. Barbaro RP. Lancet.
- Myocarditis after Covid-19 Vaccination in a Large Health Care Organization. 10/6/21. Witberg G. New Engl J.
- One-year sustained cellular and humoral immunities of COVID-19 convalescents. 10/5/21. Zhang J. Clin Infect Dis.
- Administration of Monoclonal Antibody for COVID-19 in Patient Homes. 10/14/21. Malani AN. JAMA Netw Open.
- REGEN-COV Antibody Combination and Outcomes in Outpatients with Covid-19. 9/29/21. Weinrich D. NEJM.
Newsletter Issue 104, October 11, 2021:
- Comparative Effectiveness of Moderna, Pfizer-BioNTech, and Janssen (Johnson & Johnson) Vaccines in Preventing COVID-19 Hospitalizations Among Adults Without Immunocompromising Conditions – United States, March-August 2021. 9/23/21. Self WH. MMWR Morb Mortal Wkly Rep.
This CDC study compared the real-life vaccine effectiveness (VE) against hospitalization of patients who had the three vaccines which were approved in the US. The vaccination status of 1,682 patients hospitalized with COVID-19 was compared with the vaccination status of 2,007 control patients admitted without COVID-19 during March to August 2021. VE against COVID-19 hospitalization was slightly lower for the Pfizer vaccine (88%) than the Moderna vaccine (93%), with this difference driven by a decline in VE after 120 days for the Pfizer but not for the Moderna vaccine. The Janssen (Johnson and Johnson) VE was 71%.
SAB Comment: As viral variants were not determined in this study, and time since vaccination is increasing, the VE of various vaccines may be changing. Note that in the first week of May 2021, 1.6% of all COVID-19 infections in the US were thought to be caused by Delta, whereas in the last week of September, 99% of US cases were Delta. - Myocarditis With COVID-19 mRNA Vaccines. 8/10/21. Bozkurt B. Circulation.
This report summarizes the available information regarding myocarditis occurring after mRNA vaccination against SARS-CoV-2. The CDC reports an incidence of 12.6 per million of those between ages 12 and 39, mostly men. The FDA will add a warning label to both mRNA vaccines. Case definition, symptoms, treatment, and course are presented, as well as a chart listing published cases. Rapid resolution usually occurred. The mechanism of development is unclear, but proposed mechanisms are discussed. It includes an illustration of the risk-benefit which favors vaccination for all people older than 12. - Fostamatinib for the treatment of hospitalized adults with COVID-19: A randomized trial. 9/1/21. Strich JR. Clin Infect Dis.
Fostamatinib is an oral tyrosine kinase inhibitor that is FDA-approved for the treatment of chronic idiopathic thrombocytopenic purpura. Its active metabolite inhibits both the release of proinflammatory cytokines and platelet mediated thrombus formation provoked by anti-spike immune complexes. In this NIH-led pilot study, fostamatinib was given to 30 patients with advanced COVID-19 requiring oxygen and receiving remdesivir and corticosteroids. Compared to the placebo group, lung injury appeared to resolve more quickly, serious side effects were significantly reduced, and several biomarkers improved significantly. Larger confirmatory trials are needed to establish the drug’s role in advanced SARS-CoV-2 infections. - Antifungal prophylaxis for prevention of COVID-19-associated pulmonary aspergillosis in critically ill patients: an observational study. 9/16/21. Hatzl S. Crit Care.
This retrospective, observational study reviewed the clinical course of all 132 consecutive patients admitted between September 1, 2020, and May 1, 2021, 75 of whom received antifungal prophylaxis (98% posaconazole). Antifungal prophylaxis was recommended in this medical center, but ordering it was left up to the discretion of individual intensivists. The authors noted that COVID-19-associated pulmonary aspergillosis (CAPA) was diagnosed in 17.5% of patients who did not receive antifungal prophylaxis, versus only in 1.4% of those receiving prophylaxis. They also noted that despite the efficacy shown for antifungal prophylaxis against aspergillosis infection in these patients, this prophylaxis did not have a significant impact on overall survival. - Machine Learning Prediction of Death in Critically Ill Patients With Coronavirus Disease 2019. 9/3/21. Churpek MM. Crit Care Explor.
This observational study (67 US ICUs, N=5075, March-June 2020) addressed the variable mortality of ICU/COVID-19 patients with a machine learning tool ~eXtreme Gradient Boosting (XGBM) on 28-day mortality. XGBM had the highest discrimination and calibration of all the machine learning models tested including, SOFA Score, NEWS and CURB-65. It is a simple bedside tool that provides pertinent information for goals of care discussions, triage decisions and for prognostic clinical trials. The area under the receiver operating curve was 0.81 (CI 79-85) with a discrimination power X 10 fold. Mortality was 36.4% at day 28 from day of ICU admission. Age, number of ICU beds, creatinine, and lactate were important contributions to mortality.
Newsletter Issue 103, October 4, 2021:
- Antibody and cellular therapies for treatment of covid-19: a living systematic review and network meta-analysis. 9/24/21. Siemieniuk RA. BMJ.
This updatable meta-analysis from the World Health Organization and British Medical Journal collaboration reviews the results of 47 randomized trials (selected for quality from 1050) dating to July 2021. It evaluates research on monoclonal antibodies, polyclonal antibodies, convalescent plasma, intravenous immunoglobulin (IVIg), umbilical cord mesenchymal stem cells, peripheral blood nonhematopoietic enriched stem cells, anti-SARS-CoV-2 IVIg, and therapeutic plasma exchange. The authors’ current conclusions are that in patients with nonsevere COVID-19, the antiviral monoclonal antibodies casirivimab-imdevimab (Regeneron) probably reduce risk of hospitalization, and that bamlanivimab, bamlanivimab-etesevimab (Lilly), and sotrovimab (GlaxoSmithKline) may reduce risk of hospitalization. None of the other interventions reviewed appear to have any impact when given to patients with nonsevere COVID-19, and none of the monoclonal antibodies or other therapies appear to have any impact when given to patients with severe covid-19 with the exception that casirivimab-imdevimab may reduce the risk of mortality in patients with severe COVID-19 who do not have detectable antibodies to the SARS-CoV-2 spike protein.
SAB Comment: Our policy has been to refrain from including nonpeer reviewed preprints in our newsletter. While this review article includes 10 such preprints among the 47 studies considered, we believe that those studies have essentially undergone peer review by the authors of this systematic review. - The effect of immunosuppressants on the prognosis of SARS-CoV-2 infection. 9/3/21. Ward D. Eur Respir J.
This retrospective Danish study found that pre-COVID-19 exposure to corticosteroids increased the risk of ICU admission and death in patients who developed PCR-positive COVID-19. The authors analyzed 36,737 cases of COVID-19, of which 527 were exposed to immunosuppressants within the 120 days preceding a positive PCR. For patients exposed to systemic glucocorticoids (at least 7.5mg prednisone equivalent per day), compared to unexposed patients, the adjusted risk ratio for ICU admission was 1.76 and of death was 2.38. Exposure to other immunosuppressants, including selective immunosuppressants, interleukin inhibitors, tumor necrosis factor inhibitors, calcineurin inhibitors, hydroxychloroquine and chloroquine, was not associated with an increased risk of hospitalization, ICU admission nor death.
SAB Comment: This information may be useful to providers prescribing immunosuppressive drugs during the pandemic. Though the use of steroids is associated with worse COVID-19 disease, this study is not designed to determine if reducing steroid dose is helpful. - Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: a double-blind, randomized controlled phase 3 trial. 9/4/21. Kyriazopoulou E. Nat Med.
From December 2020 to March 2021, 594 COVID-19 positive patients at 30 study sites underwent a phase 3 double-blinded randomized controlled trial evaluating the efficacy and safety of early initiation of anakinra treatment on the WHO clinical progression scale (WHO-CPS) at day 28. The study patients (405 anakinra and 189 placebo) all had a soluble urokinase plasminogen activator receptor (suPAR) titer >6ng/ml, which is an early predictor of severe respiratory failure. At 28 days, the odds ratio of having a worse clinical status on the WHO-CPS was 0.36 in the anakinra group. The study includes consideration of CRP, neutrophil to lymphocyte ratio, ferritin, AST and IL-6 levels as well as SOFA scores. - Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. 9/4/21. Marconi VC. Lancet Respir Med.
This oral Janus kinase inhibitor with anti-cytokine properties and potential antiviral effectiveness has been studied extensively in hospitalized patients with COVID-19. Sponsored by Pfizer, the COV-BARRIER trial enrolled 1525 participants from 101 centers in 12 countries between June 2020 and January 2021. While its ability to reduce disease progression was not significant, 28-day all-cause mortality was reduced by 5% (from 13% to 8%) in the baricitinib group compared to the placebo population (HR 0.57) which translates in one additional death prevented per 20 baricitinib-treated patients. An accompanying editorial highlights the effectiveness and safety of JAK inhibitors in combination with steroids and remdesivir. It also provides plausible explanations for the observed differences in the results of this trial (COV-BARRIER) and the ACCT-2 trial of baricitinib. - Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): a phase 3, randomised, controlled, open-label trial. 9/17/21. Ader F. Lancet Infect Dis.
DisCoVeRy is a phase 3, open-label, adaptive, multicenter, randomized, controlled trial conducted in 48 sites in Europe, studying 857 COVID-19 patients. Four hundred twenty-nine patients were admitted with mild to moderate symptoms (WHO scale of more than 3 and less than 6), had symptoms for more than 7 days, and who were on oxygen prescription were treated with remdesivir, while 428 patients were treated with standard care. Outcome were measured at days 3 through 29. No clinical benefit regarding viral clearance, mortality, morbidity or recovery was noted with remdesivir. - Additional new articles selected for the IARS COVID-19 Resource website and searchable by topic:
- Clinical Characteristics of Multisystem Inflammatory Syndrome in Adults: A Systematic Review. 9/22/21. Patel P. JAMA Netw Open.
- Comparative Effectiveness of Moderna, Pfizer-BioNTech, and Janssen (Johnson & Johnson) Vaccines in Preventing COVID-19 Hospitalizations Among Adults Without Immunocompromising Conditions – United States, March-August 2021. 9/23/21. Self WH. MMWR Morb Mortal Wkly Rep.
- Myocarditis With COVID-19 mRNA Vaccines. 8/10/21. Bozkurt B. Circulation.
- Antifungal prophylaxis for prevention of COVID-19-associated pulmonary aspergillosis in critically ill patients: an observational study. 9/16/21. Hatzl S. Crit Care.
- Fostamatinib for the treatment of hospitalized adults with COVD-19: A randomized trial. 9/1/21. Strich JR. Clin Infect Dis.
- Machine Learning Prediction of Death in Critically Ill Patients With Coronavirus Disease 2019. 9/3/21. Churpek MM. Crit Care Explor.
- Convalescent plasma for COVID-19: a meta-analysis, trial sequential analysis, and meta-regression. 9/28/21. Snow TAC. Br J Anaesth.
Newsletter Issue 102, September 27, 2021:
- Protection of BNT162b2 Vaccine Booster against Covid-19 in Israel. 9/15/21. Bar-On YM. N Engl J Med.
More than 1.1 million (1,137,804) fully vaccinated Israelis older than 60 years were studied during the rollout of a program to provide 3rd shots of the Pfizer vaccine during the Delta surge. Data was analyzed from 5 million person-days at risk in the nonbooster group compared with 10 million person-days in the booster group. “At least 12 days after the booster dose, the rate of confirmed infection was lower in the booster group than in the nonbooster group by a factor of 11.3 (95% confidence interval [CI], 10.4 to 12.3); the rate of severe illness (cases diagnosed Aug 10-26, 2021) was lower by a factor of 19.5 (95% CI, 12.9 to 29.5).” A second analysis showed that the reduction after 12 days post 3rd shot was 5.4 times greater than the reduction after 4 to 6 days post-3rd shot. (95% CI, 4.8 to 6.1). A bar graph shows clearly that it takes 2-3 weeks for additional protection to peak. - Safety and immunogenicity of SARS-CoV-2 variant mRNA vaccine boosters in healthy adults: an interim analysis. 9/16/21. Choi A. Nat Med.
In an open-label ongoing phase 2a study, Moderna examined whether their current vaccine (mRNA-1273) booster 6 months after the second dose, shows decreased neutralization vs. three Beta-variant vaccines. Interim analysis of 4 booster groups (n = 20/group) is: Pre-booster dose: neutralizing antibodies against wild-type D614G waned vs. peak titers 1-month post-primary series. Neutralization titers against Beta, Gamma and Delta VOCs were low/undetectable. Both the mRNA-1273 booster and variant-modified boosters were safe. Both boosters increased neutralization titers against wild-type D614G vs. peak titers 1 month after the primary series, and importantly, against VOCs; both were equivalent or superior to titers measured post-primary series against wild-type virus. - SARS-CoV-2 Neutralization with BNT162b2 Vaccine Dose 3. 9/15/21. Falsey AR. N Engl J Med.
This research letter discusses what amounts to a pilot study looking at neutralizing antibody responses in a small group of subjects who received a third Pfizer vaccine dose, providing data that may be used to argue for a booster. Increases were greater in participants older than 65 years compared with adults younger than 55. Increases were greater to Beta and Delta variants than to wild type. - Spontaneous Abortion Following COVID-19 Vaccination During Pregnancy. 9/8/21. Kharbanda EO. JAMA.
In this Research Letter of 105,446 pregnancies with 13,160 spontaneous abortions, vaccination for COVID-19 did not increase the risk for spontaneous abortion as compared to unvaccinated pregnancies. - Effectiveness of the BNT162b2 mRNA COVID-19 vaccine in pregnancy. 9/8/21. Dagan N. Nat Med.
A pre-Delta, observational study from researchers in Tel Aviv and at Harvard investigating the BNT162b2 messenger RNA vaccine during pregnancy in Israeli women older than 16 years found the vaccine to be of comparable effectiveness to the general population and that it reduced the infection rate by nearly 50% (see data in Figure 1) when compared to the unvaccinated pregnant control group. It should be noted there were no deaths in either group and only 1 severe infection in the unvaccinated group. The authors hypothesize the vaccination is safe and might provide protection in newborns, although they offered no evidence. - Surveillance for Adverse Events After COVID-19 mRNA Vaccination. 9/3/21. Klein NP. JAMA.
This is a Vaccine Safety Datalink study from 8 participating US health plans. “In this interim analysis of surveillance data from 6.2 million persons who received 11.8 million doses of an mRNA vaccine, event rates for 23 serious health outcomes were not significantly higher for individuals 1 to 21 days after vaccination compared with similar individuals at 22 to 42 days after vaccination,…although CIs were wide for some rate ratio estimates and additional follow-up is ongoing.” Outcomes included MI, Bell palsy, cerebral venous sinus thrombosis, Guillain-Barré, myocarditis, pericarditis, PE, CVA, and thrombosis with thrombocytopenia. Follow-up is expected for at least 2 years. - Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine through 6 Months. 9/15/21. Thomas SJ. N Engl J Med.
This article updates the 2-month data from the ongoing randomized, placebo controlled study of 44,165 participants older than 16 years and 2,264 participants 12-15 years old who received 2 doses of BNT162b2 or placebo. Vaccinations were pre-Delta.
Newsletter Issue 101, September 20, 2021:
- High-Flow Nasal Cannula and COVID-19: A Clinical Review. 9/15/21. Crimi C. Respir Care.
This review is a succinct and thorough description of the value of high flow nasal cannula (HFNC) for moderate-to-severe acute hypoxemic respiratory failure. The published studies on the use of HFNC during the pandemic are reviewed, and ongoing randomized clinical trials are described. Evidence to date finds HFNC (generally 50-60 l/min) to be a valuable and feasible treatment option for patients with COVID-19 pneumonia, with clinical advantages (i.e., it is simple, well tolerated, available outside the ICU, and likely effective at reducing intubations). The current evidence shows that HFNC is no worse than conventional oxygen delivery devices or NIV in terms of dispersion of patient-generated bioaerosols. - The use of head helmets to deliver noninvasive ventilatory support: a comprehensive review of technical aspects and clinical findings. 9/9/21. Coppadoro A. Crit Care.
Helmets have been in use since the early 2000s albeit mostly in a few countries, particularly Italy. Their use has increased with the COVID-19 epidemic. This excellent, brief review notes that helmet therapy can be safely and effectively used to provide NIV (non-invasive ventilation) during hypoxemic respiratory failure, improving oxygenation better than standard oxygen mask treatment and possibly leading to superior patient-centered outcomes than other NIV interfaces. The authors point out issues related to helmet NPPV (non-invasive positive-pressure ventilation) including slow helmet pressurization, reduced CO2 washout and patient–ventilator asynchrony. They state, however, that helmet NPPV is superior to face mask NPPV for patients with ARDS, can be successfully used to treat hypoxemia and is an excellent mode for immunocompromised patients. Helmet NPPV is, however, inferior in patients with COPD exacerbations. - Investigation of the optimal method of oxygen administration with simultaneous use of a surgical mask: a randomized control study. 9/7/21. Matsui Y. J Anesth.
In this small (n=24) healthy volunteer study, three methods to deliver supplemental oxygen along with surgical masking were assessed by oxygen reserve index* (ORi) and end-tidal oxygen (EtO2). The goal was to determine the best method to limit aerosol dispersal while transporting patients, using 4 L/M supplemental O2 for consistency. A surgical mask over nasal cannula provided the best results of the 3 methods tested, resulting in an ORi of 0.50 and EtO2 of 33%. The other tested methods were oxygen mask over surgical mask and oxygen mask under surgical mask.
*SAB Comment: ORi is a relatively new method using multi-wavelength co-oximetry. Incorporated within some pulse oximeters, it provides a noninvasive method to assess changes in the moderately hyperoxic range, (PaO2100-200 mmHg) one within which SpO2 is insensitive. With decreasing PaO2, ORi will decline before SpO2. - Additional new articles selected for the IARS COVID-19 Resource website and searchable by topic:
- Convalescent plasma for hospitalized patients with COVID-19: an open-label, randomized controlled trial. 9/10/21. Bégin P. Nat Med.
- Results of the CAPSID randomized trial for high-dose convalescent plasma in severe COVID-19 patients. 8/31/21. Körper S. J Clin Invest.
- Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: a double-blind, randomized controlled phase 3 trial. 9/4/21. Kyriazopoulou E. Nat Med.
- The effect of immunosuppressants on the prognosis of SARS-CoV-2 infection. 9/3/21. Ward D. Eur Respir J.
- SARS-CoV-2 B.1.617.2 Delta variant replication and immune evasion. 9/6/21. Mlcochova P. Nature.
- Machine Learning Prediction of Death in Critically Ill Patients With Coronavirus Disease 2019. 9/3/21. Churpek MM. Crit Care Explor.
- Evolution of COVID-19 symptoms during the first 12 months after illness onset. 9/2/21. Wynberg E. Clin Infect Dis.
Newsletter Issue 100, September 13, 2021:
- Hospital admission and emergency care attendance risk for SARS-CoV-2 delta (B.1.617.2) compared with alpha (B.1.1.7) variants of concern: a cohort study. 8/27/21. Twohig KA. Lancet Infect Dis.
All English National Health System patients diagnosed with COVID-19 by PCR from March 29 – May 23, 2021, and found by whole-genome sequencing to have alpha or delta variants, were studied. Delta grew from 0.1% to 45.8% during the study. The adjusted risk (aHR) of an emergency care visit or hospital admission within 14 days of a first positive test was 1.45 in those with delta (n=8,682) compared with alpha (n=34,656). For hospital admission, aHR for delta vs. alpha was 2.26. Median age was 31 years old; 74% were unvaccinated in both variant groups. Patients seen in emergency care or admitted on the day of their first COVID-positive test were excluded to reduce bias of screening tests at the time of presentation for non-COVID related illness. - 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. 8/28/21. Huang L. Lancet.
This extensive study reports the condition at 12 months of a cohort of 1,307 COVID-19 patients discharged January-May 2020 from a single hospital in China. Patients in nursing or care homes, immobile or with osteoarthritis, and with psychiatric disorders or dementia were excluded from the study. A review of the report of their condition at 6 months appears in Newsletter 51. Intensive evaluations included multiple standardized questionnaires, physical exam, blood tests, pulmonary evaluation, use of healthcare resources and work status. Patients with at least one persistent symptom decreased from 68% at 6 months to 49% at 12 months. The most common problem, fatigue and muscle weakness decreased from 52% to 20%. The proportion with dyspnea and anxiety or depression worsened slightly. Of those who were employed prior to hospitalization, 88% had returned to work. Outcome with regard to severity of initial disease, males vs. females and patients vs. matched community controls is characterized. - Awake prone positioning for COVID-19 acute hypoxaemic respiratory failure: a randomised, controlled, multinational, open-label meta-trial. 8/23/21. Ehrmann S. Lancet Respir Med.
In this multicenter, international, randomized, open-label meta-trial, awake prone positioning (APP) decreased the incidence of intubation in patients with acute severe hypoxemic respiratory failure due to COVID-19 supported with high-flow nasal cannula. From April 2020 through January 2021, 1,121 patients from six countries were randomized to APP as long as possible, or to standard care. The number needed to treat with APP to prevent one intubation was 14. Though not designed to evaluate the duration of APP on outcomes (median daily duration was 5 hours), patients achieving longer durations had better outcomes. Adverse effects were mild, infrequent, and occurred at similar rates between the APP and standard care groups.
SAB Comment: This is the first large, randomized study of APP, commonly used empirically during the pandemic. The results reinforce the safety and utility of APP for averting intubations. Other randomized studies are underway, as discussed in the accompanying Comment. - COVID-19 Vaccine Safety in Adolescents Aged 12-17 Years – United States, December 14, 2020-July 16, 2021. 8/5/21. Hause AM. MMWR Morb Mortal Wkly Rep.
A statistical analysis of the Pfizer COVID-19 vaccine in children 12 years or older in the US demonstrated its safety. Reactions to the vaccine are uncommon and mostly mild. Myocarditis is one rare but severe reaction more common in boys after the second vaccination and that resulted in no deaths. - COVID-19 Vaccination-Associated Myocarditis in Adolescents. 8/14/21. Jain SS. Pediatrics.
This article reviews the clinical presentation and early prognosis of the rare complication of acute myocarditis following COVID-19 vaccination in adolescents. The authors pool data from 63 patients from 16 US institutions. Using cardiac MR imaging, the authors are able to characterize this entity with exquisite detail in the figures. The authors demonstrate the favorable short-term outcomes of this subset. This article represents some of the largest dataset examining this particular entity in this age group. - Safety of the BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Setting. 8/25/21. Barda N. N Engl J Med.
This Israeli study compared the occurrence of adverse events in 884,828 recipients of the Pfizer/BioNTech COVID-19 vaccine to a like number of controls matched for risks on the day of vaccination. Vaccine recipients and controls were followed for 21 days after each injection. Vaccination was not associated with an elevated risk of most of the adverse events examined. Vaccination was associated with an elevated risk of myocarditis (risk ratio, 3.24 but absolute event rate only 2.23 per 100,000), lymphadenopathy (risk ratio, 2.43), appendicitis (risk ratio, 1.40), and herpes zoster infection (risk ratio, 1.43). From a second set of data, they showed that actual SARS-CoV-2 infection was associated with a substantially increased risk of myocarditis (risk ratio, 18.28) and with additional serious adverse events, including pericarditis, arrhythmia, deep-vein thrombosis, pulmonary embolism, myocardial infarction, intracranial hemorrhage, and thrombocytopenia.
SAB Comment: To make meaning of a comparison of adverse events associated with vaccination to those associated with COVID-19 infection, one must assume a cumulative incidence level. The accompanying editorial adds context and assumes that, “given the current state of the global pandemic, however, the risk of exposure to SARS-CoV-2 appears to be inevitable.” - Effectiveness of COVID-19 Vaccines in Preventing SARS-CoV-2 Infection Among Frontline Workers Before and During B.1.617.2 (Delta) Variant Predominance – Eight U.S. Locations, December 2020-August 2021. 8/26/21. Fowlkes A. MMWR Morb Mortal Wkly Rep.
Data from the prospective frontline worker HEROES-RECOVER Cohorts showed that from 12/24/20-4/10/21 the Pfizer-BioNTech and Moderna vaccines were ~90% effective in preventing symptomatic and asymptomatic SARS-CoV-2 infection. Adjusted efficacy was 80%. The estimate was 85% among participants for whom less than 120 days had elapsed since full vaccination and 73% among those for whom 150 or more days had elapsed. Once Delta became the predominant variant, adjusted efficacy decreased from 91% to 66%. However, this trend should be interpreted with caution as effectiveness might also have declined due to greater time since vaccination. In addition, there were few weeks of observation and low numbers of infections. - Early Convalescent Plasma for High-Risk Outpatients with Covid-19. 8/18/21. Korley FK. N Engl J Med.
While prior studies using convalescent plasma have failed to demonstrate improved outcomes over placebo for inpatients, this randomized study examined its use in outpatients. Patients older than 50 years old were initially seen in the emergency room and diagnosed with COVID-19. Five hundred and eleven patients from 48 hospitals in 21 states in the US were included in this blinded study funded by the NIH, 257 received convalescent plasma and 254 received placebo. The primary outcome was disease progression defined by either hospital admission, seeking emergency or urgent care, or death. The study showed no significant difference between the two groups (i.e., those receiving convalescent plasma and those that did not).
Newsletter Issue 99, September 8, 2021:
- An outbreak caused by the SARS-CoV-2 Delta variant (B.1.617.2) in a secondary care hospital in Finland, May 2021. 7/29/21. Hetemäki I. Eurosurveillance.
Authors describe a Delta variant outbreak that originated from one inpatient. It spread within the hospital and to three primary care facilities. Fifty-eight patients and 45 healthcare workers became infected; 18 elderly patients admitted with other conditions died following infection. Among infected individuals, 19% were fully vaccinated, 47% had 1 dose and 34% were unvaccinated. Over 90% of vaccinations were with BioNTech-Pfizer vaccine. Both symptomatic and asymptomatic infections were found among vaccinated healthcare workers, and secondary transmission occurred from patients with symptomatic infections despite use of PPE. Presymptomatic and asymptomatic individuals also infected others. Authors recommend FFP2/3 respirators (~N95 or higher grade) while treating COVID-19 patients.
SAB Comment: The above report, based upon careful contact tracing, highlights the value of universal masking with respirators of FFP2 of N95-level or greater when caring for COVID-19 patients. - Durability of mRNA-1273 vaccine–induced antibodies against SARS-CoV-2 variants. 8/12/21. Pegu A. Science.
The authors assess the impact of SARS-CoV-2 variants B.1.1.7 (Alpha), B.1.351 (Beta), P.1 (Gamma), B.1.429 (Epsilon), B.1.526 (Iota), and B.1.617.2 (Delta) on binding, neutralizing, and ACE2-competing antibodies elicited by the Moderna mRNA-1273 vaccine over seven months. Cross-reactive neutralizing responses were rare after a single dose. At the peak of response to the second vaccine dose, all individuals had responses to all variants. Binding and functional antibodies against variants persisted in most subjects, albeit at low levels, for 6 months after the primary series of the mRNA-1273 vaccine. Across all assays, B.1.351 had the lowest antibody recognition. The authors believe that these data complement ongoing studies to inform the potential need for additional boost vaccinations. - SARS-CoV-2 infection and venous thromboembolism after surgery: an international prospective cohort study. 8/24/21. COVIDSurg Collaborative. Anaesthesia.
This is a planned sub-study and analysis from a prospective, international, multicenter cohort study of patients undergoing all surgical procedures during October 2020 to determine incidence of postoperative venous thromboembolism (VTE) in patients without and with history of SARS-CoV-2 (before [more than 7 weeks]; recent [1-6 weeks]; peri-operative [7 days before to 30 days after]). Primary outcome measure VTE (PE or DVT) was within 30 days. No information on VTE prophylaxis pre- or post-operatively was available. VTE was independently associated with postoperative mortality (5.4%). In patients with SARS-CoV-2 mortality without VTE was 7.4%; with VTE was 40.8%. Recent and peri-operative SARS-CoV-2 infection may be an independent risk factor for postoperative VTE, and increased awareness and surveillance should be considered. - The COVID-19 SAB has added the following articles to the COVID-19 Resource website in the last week:
- Association of in-hospital use of ACE-I/ARB and COVID-19 outcomes in African American population. 8/19/21. Li S. J Clin Invest.
- COVID-19 Vaccine Safety in Adolescents Aged 12-17 Years – United States, December 14, 2020-July 16, 2021. 8/5/21. Hause AM. MMWR Morb Mortal Wkly Rep.
- Effect of anakinra on mortality in patients with COVID-19: a systematic review and patient-level meta-analysis. 8/16/21. Kyriazopoulou E. Lancet Rheumatol.
- SARS-CoV-2 variant prediction and antiviral drug design are enabled by RBD in vitro evolution. 8/16/21. Zahradník J. Nature Microbiology. https://www.nature.com/articles/s41564-021-00954-4.
Newsletter Issue 98, August 30, 2021:
Author’s Note on today’s Special Edition summary on “The Virus, The Vaccines and The Variants”: This review cites published peer-reviewed articles or official governmental websites only. The information is current as of August 27, 2021. Given the fluid, constantly developing nature of the pandemic and its management, we intend to provide regular updates to this summary for our readers.
The Virus, The Vaccines and The Variants
Robert N. Sladen MBChB, FCCM, IARS Scientific Advisory Board
The Virus
The coronavirus SARS-CoV-2 shares 80% of the genome sequence of SARS-CoV-1 (responsible for the SARS epidemic in 2002-3) and in its original, “wild” form is less lethal but far more transmissible1. Each spherical virion incorporates four structural proteins: a central nucleocapsid containing single-stranded RNA, a membrane, an envelope and a corona of spike (S) proteins. A short 25-amino acid sequence on the S1 subunit, the outer component of the S-protein, forms the viral receptor binding domain (RBD) that binds with ACE2 receptors and triggers cellular entry and viral replication. ACE2 is ubiquitous in multiple organs, converts angiotensin I to angiotensin (1-7), and provides endothelial protection. Viral blockade of the ACE2 receptor contributes to many of the vascular, coagulopathic and cytopathologic manifestations of COVID-192.
The Vaccines
Vaccines in current use against SARS-CoV-2 induce neutralizing antibodies (nAbs) that bind to the viral RBD and block its interaction with the ACE2 receptor (Table 1). Vaccine efficacy is determined by randomized controlled trials but these do not always predict effectiveness under nonrandom field conditions in heterogenous populations and geographic zones3. Levels of nAbs are often used as a surrogate for outcomes but their relationship with vaccine effectiveness is still incompletely understood. Although all vaccines currently in use in the USA remain highly effective in preventing severe illness, hospitalization and death, there is increasing evidence that asymptomatic or presymptomatic vaccinated individuals can become infected, shed and transmit active virus2.
Table 1: Vaccines Currently in Use or in Phase III Trials in the USA
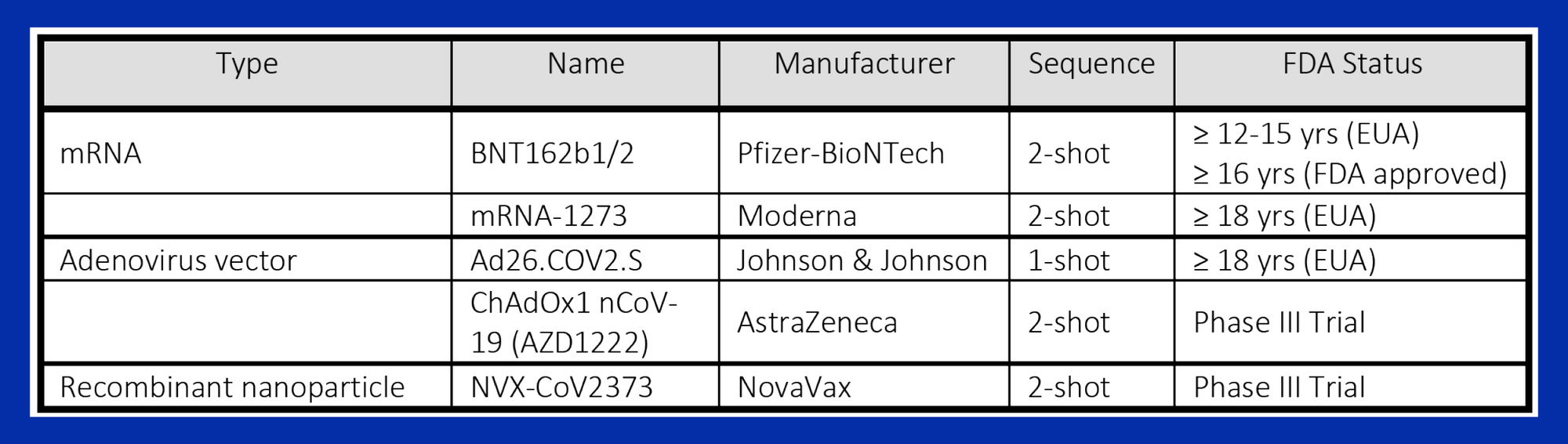
The FDA granted emergency use authorization (EUA) to the Pfizer-BioNTech, Moderna and Johnson & Johnson (J&J) vaccines for the duration of the COVID-19 pandemic. None have been approved for children younger than 12 years. Only the Pfizer-BioNTech vaccine has been approved for adolescents younger than 18 years of age, and on August 23, 2021, it received full FDA approval for use in individuals aged 16 years or older. The AstraZeneca vaccine is used widely in countries outside the US but received a major setback by reports of the rare but potentially fatal complication of vaccine-induced immune thrombotic thrombocytopenia (VITT); the J&J vaccine was briefly suspended in the US during investigation of similar reports. The unique NovaVax vaccine is undergoing Phase III trials that have been set back by FDA concerns regarding its manufacturing process.
The Variants
Mutations and Variant Categories
All viruses constantly undergo adaptive mutations to their genome to form variants. Coronaviruses are singular in having the largest genome of all RNA-based viruses2, although they mutate five-fold more slowly than influenza viruses4. A variant may have multiple or even a single mutation in the S-protein sequence and although most are of little clinical or public health consequence, minimal genomic sequence alterations of the small RBD can lead to immune escape. Important variants may emerge in a single individual, especially those who are immunocompromised and maintain high viral loads over a protracted length of time4. Stochastic modeling predicts that the highest risk of emergence of resistant variants occurs when there is a combination of high infection with low vaccination rate, but also when a high proportion of the population is vaccinated but transmission is not controlled because of a reversion to prepandemic behavior5. New variants that are more transmissible and virulent are constantly evolving and “pushing out” older strains.
The highly complex systems of nomenclature attempt to group clades of variants by their genome and lineage on the phylogenetic tree. The PANGOLIN classification (Phylogenetic Assignment of Named Global Outbreak Lineages) has become well established (cov-lineages.org), but the World Health Organization (WHO) has attempted to simplify nomenclature by grouping variant strains by letters of the Greek alphabet. The US Government SARS-CoV-2 Interagency Group (SIG) has defined three phenotypical categories of variants.
Variants of concern (VOCs) (Table 2) are strains associated with an established detriment to human health through increased transmissibility, immune evasion/escape and virulence. They may evade detection by RT-PCR testing and potentially cause reinfection and vaccine breakthrough6. Variants that increase the affinity of the viral spike protein for ACE2 receptors enhance viral attachment, cellular entry and create faster replication rates. Some mutations exist in several VOCs. The E484K “escape” mutation involves an S-protein side chain charge change, and has been observed in the alpha, beta and gamma variants and markedly increases immune resistance7.
Table 2: Current Variants of Concern (VOCs) in the USA
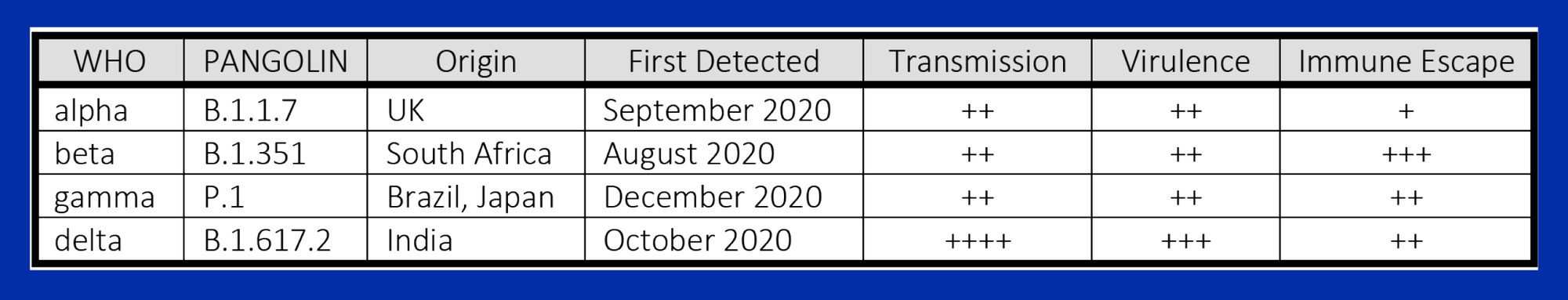
Variants of Interest (VOI) have some of the characteristics of VOCs but limited prevalence at present and include epsilon, zeta, eta, iota, theta, kappa and lambda. The epsilon variant (B.1.427) was first detected in the US in June 2020 and declared a VOC but de-escalated to VOI on June 29, 2021 because of vaccine effectivity and a marked decline in its prevalence. The lambda variant (C.37) was first described in Peru in December 2020, now dominates in South America and provides concern for its high virulence and immune escape from current vaccines.
Variants of High Consequence (VOHC) are variants with even greater virulence and immune escape than existing VOCs. Currently no variants are listed in this class in the US.
Variant Outbreaks
In late 2020 and early 2021, variants caused severe resurgent outbreaks in many parts of the world including the UK, India, South Africa and Peru8. In April-May 2021, the delta variant emerged in India and contributed to a massive second wave9. Subsequently it has triggered a major resurgence of COVID-19 cases in the US, particularly in states with low proportions of vaccinated individuals10. It is more than twice as contagious as previous strains and appears to cause more rapidly progressive disease and hospitalizations in unvaccinated patients, including young adults and children. Vaccinated individuals are at a greatly decreased risk of contracting severe disease but there is increasing recognition of their susceptibility to asymptomatic COVID-19, and even though their viral load declines rapidly, they may thereby infect susceptible persons.
The Centers for Disease Control and Prevention (CDC) website11 provides an informative link to its genomic surveillance program. It also has a model (Nowcast) that calculates the rapidly changing proportion of variants causing COVID-19 infection by geographic region. For example, in the first week of May 2021 1.6% of all COVID-19 infections in the US were projected to be caused by the delta variant. By the first week of August this had increased to 96.8%.
Variant Vaccine Resistance
Initial studies showed high effectiveness of mRNA and virus vector vaccines versus the wild type virus, and the AstraZeneca vaccine was shown to decrease viral load and duration of shedding of the alpha variant12. However, efficacy changes with geographic variant predominance. In 2020, the AstraZeneca vaccine had 74% efficacy in the UK13, but only 22% in South Africa, where the beta variant had become dominant14. Studies from Qatar with the Pfizer-BioNTech vaccine showed decreased effectiveness against infection by the alpha (95% to 89.5%) and beta variants (95% to 75%), but high protection (97.4%) persisted against severe, critical or fatal disease 14 days or more after a second dose15. In a UK observational study of almost 20,000 symptomatic genome sequenced cases, effectiveness of one dose of the Pfizer-BioNTech and AstraZeneca vaccines was 48.7% against the alpha variant but only 30.7% against the delta variant16. After two doses, the effectiveness of the Pfizer-BioNTech against the alpha and delta variants increased to 93.7% and 88.0% respectively, and for the AstraZeneca vaccine 74.5% and 67.0% respectively.
Vaccine Breakthrough Infections and Boosters
Breakthrough infections were first reported in two fully mRNA vaccinated individuals in New York in March 2021 despite a brisk neutralizing antibody response17. An epidemiologic study in Washington state prior to the delta surge revealed that 24 of 1547 US Military Health System beneficiaries – mostly healthy young healthcare workers – had positive RT-PCR 14 days or later after full vaccination18. Most had mild symptoms but some had considerable viral shedding.
By mid-March 2021, more than 80% of the adult population of Israel had been given at least one dose of the Pfizer-BioNtech vaccine and national surveillance data revealed 95% effectiveness against SARS-CoV-2 infection and 97% effectiveness against hospitalization or death19. Shortly after a winter surge when the alpha variant occurred in 94.5% of isolates, breakthrough infections were detected in 2.6% of fully vaccinated health care workers. Although the majority had no or mild symptoms, in 19%, symptoms persisted more than 6 weeks20. There is evidence that nAbs decline with duration after vaccination with Pfizer-BioNTech regimen, especially in older individuals21. After detecting an increasing number of delta variant breakthrough infections in fully vaccinated individuals, on August 13, 2021 the Israel Ministry of Health approved a third vaccine for persons 50 years old or older, healthcare workers and other high-risk groups22. On the same day, the CDC recommended a third dose for moderately to severely immunocompromised individuals23 and the Biden Administration has recommended a third dose of the Pfizer-BioNTech or Moderna vaccine starting this autumn.
Future Directions: Can We Bring the Pandemic to an End?
High transmission with low vaccination rates continually creates opportunities for the emergence of new variants with unpredictable degrees of immune escape. This emphasizes the importance of genomic sequencing to detect and quantify the predominance of variants. It is suggested that the pandemic will likely end only when vaccines against circulating variants are delivered equitably around the world24.
On the other hand, vaccines active only against the S-protein RBD may promote the emergence of more virulent escape-mutations by natural selection25. Infection of a vaccinated individual by mutated and nonmutated virions could foster selective replication of the mutated virions. This suggests that second generation vaccines should be polyvalent and act on multiple epitopes of the SARS-CoV-2 virus. For example, the VXA-CoV2-1 (Vaxart) vaccine that is currently undergoing Phase II trials in the US is being developed as an oral vaccine directed against the viral nucleocapsid (N-protein), making it potentially less susceptible to mutations.
All zoonotic sarbecoviruses use human ACE2 as the entry receptor, so it is suggested that third generation vaccines should inhibit the RBD-ACE2 interaction without blocking beneficial ACE2 activity. In SARS survivors, who continue to have detectable nAbs to SARS-CoV-1 17 years after infection, the Pfizer-BioNTech vaccine induced potent cross-clade pan-sarbecovirus nAbs, capable of neutralizing known VOCs as well as sarbecoviruses that exist in bats and pangolins with the potential for human infection1. A humanized ACE2 monoclonal targeted nAb (h11B11) provides protection against SARS-CoV-2 and escape variants in animal models26, which have also suggested that a cocktail combining ACE2 protecting peptides and S-protein neutralizing peptides may be safe and effective27.
References
- Tan CW, Chia WN, Young BE, Zhu F, Lim BL, Sia WR, et al. Pan-Sarbecovirus Neutralizing Antibodies in BNT162b2-Immunized SARS-CoV-1 Survivors. N Engl J Med. 2021. 10.1056/NEJMoa2108453.
- Forchette L, Sebastian W, Liu T. A Comprehensive Review of COVID-19 Virology, Vaccines, Variants, and Therapeutics. Curr Med Sci. 2021. 10.1007/s11596-021-2395-1.
- Hodgson SH, Mansatta K, Mallett G, Harris V, Emary KRW, Pollard AJ. What defines an efficacious COVID-19 vaccine? A review of the challenges assessing the clinical efficacy of vaccines against SARS-CoV-2. Lancet Infect Dis. 2021;21:e26-e35. 10.1016/S1473-3099(20)30773-8.
- Otto SP, Day T, Arino J, Colijn C, Dushoff J, Li M, et al. The origins and potential future of SARS-CoV-2 variants of concern in the evolving COVID-19 pandemic. Curr Biol. 2021;31:R918-R29. 10.1016/j.cub.2021.06.049.
- Rella SA, Kulikova YA, Dermitzakis ET, Kondrashov FA. Rates of SARS-CoV-2 transmission and vaccination impact the fate of vaccine-resistant strains. Sci Rep. 2021;11:15729. 10.1038/s41598-021-95025-3.
- Vasireddy D, Vanaparthy R, Mohan G, Malayala SV, Atluri P. Review of COVID-19 Variants and COVID-19 Vaccine Efficacy: What the Clinician Should Know? J Clin Med Res. 2021;13:317-25. 10.14740/jocmr4518.
- Wise J. Covid-19: The E484K mutation and the risks it poses. BMJ. 2021;372:n359. 10.1136/bmj.n359.
- Chadha J, Khullar L, Mittal N. Facing the wrath of enigmatic mutations: A review on the emergence of SARS-CoV-2 variants amid COVID-19 pandemic. Environ Microbiol. 2021. 10.1111/1462-2920.15687.
- Kunal S, Aditi, Gupta K, Ish P. COVID-19 variants in India: Potential role in second wave and impact on vaccination. Heart Lung. 2021;50:784-7. 10.1016/j.hrtlng.2021.05.008.
- Centers for Disease Control and Prevention. Delta Variant: What We Know About the Science. Updated August 26, 2021. Access August 30, 2021. https://www.cdc.gov/coronavirus/2019-ncov/variants/delta-variant.html.
- Centers for Disease Control and Prevention. SARS-CoV-2 Variant Classifications and Definitions. Updated August 24, 2021. Accessed August 30, 2021. https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-info.html.
- Emary KRW, Golubchik T, Aley PK, Ariani CV, Angus B, Bibi S, et al. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): an exploratory analysis of a randomised controlled trial. Lancet. 2021;397:1351-62. 10.1016/S0140-6736(21)00628-0.
- Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99-111. 10.1016/S0140-6736(20)32661-1.
- Madhi SA, Baillie V, Cutland CL, Voysey M, Koen AL, Fairlie L, et al. Efficacy of the ChAdOx1 nCoV-19 Covid-19 Vaccine against the B.1.351 Variant. N Engl J Med. 2021;384:1885-98. 10.1056/NEJMoa2102214.
- Abu-Raddad LJ, Chemaitelly H, Butt AA, National Study Group for C-V. Effectiveness of the BNT162b2 Covid-19 Vaccine against the B.1.1.7 and B.1.351 Variants. N Engl J Med. 2021;385:187-9. 10.1056/NEJMc2104974.
- Lopez Bernal J, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, et al. Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant. N Engl J Med. 2021. 10.1056/NEJMoa2108891.
- Hacisuleyman E, Hale C, Saito Y, Blachere NE, Bergh M, Conlon EG, et al. Vaccine Breakthrough Infections with SARS-CoV-2 Variants. N Engl J Med. 2021;384:2212-8. 10.1056/NEJMoa2105000.
- Pollett SD, Richard SA, Fries AC, Simons MP, Mende K, Lalani T, et al. The SARS-CoV-2 mRNA vaccine breakthrough infection phenotype includes significant symptoms, live virus shedding, and viral genetic diversity. Clin Infect Dis. 2021. 10.1093/cid/ciab543.
- Haas EJ, Angulo FJ, McLaughlin JM, Anis E, Singer SR, Khan F, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397:1819-29. 10.1016/S0140-6736(21)00947-8.
- Bergwerk M, Gonen T, Lustig Y, Amit S, Lipsitch M, Cohen C, et al. Covid-19 Breakthrough Infections in Vaccinated Health Care Workers. N Engl J Med. 2021. 10.1056/NEJMoa2109072.
- Wall EC, Wu M, Harvey R, Kelly G, Warchal S, Sawyer C, et al. Neutralising antibody activity against SARS-CoV-2 VOCs B.1.617.2 and B.1.351 by BNT162b2 vaccination. Lancet. 2021;397:2331-3. 10.1016/S0140-6736(21)01290-3.
- Ministry of Health. The Ministry of Health Director General Has Approved the Recommendation to Administer a Third Vaccine Doe to 50-Year-Olds and Older and to Other Populations. Updated August 13, 2021. Accessed August 30, 2021. https://www.gov.il/en/departments/news/13082021-01.
- Centers for Disease Control and Prevention. Media Statement from CDC Director Rochelle P. Walensky, MD, MPH, on Signing the Advisory Committee on Immunization Practices’ Recommendation for an Additional Dose of an mRNA COVID-19 Vaccine in Moderately to Severely Immunocompromised People. Updated August 13, 2021. Accessed August 30, 2021. https://www.cdc.gov/media/releases/2021/s0813-additional-mRNA-mrna-dose.html.
- Fontanet A, Autran B, Lina B, Kieny MP, Karim SSA, Sridhar D. SARS-CoV-2 variants and ending the COVID-19 pandemic. Lancet. 2021;397:952-4. 10.1016/S0140-6736(21)00370-6.
- Galili U. COVID-19 variants as moving targets and how to stop them by glycoengineered whole-virus vaccines. Virulence. 2021;12:1717-20. 10.1080/21505594.2021.1939924.
- Du Y, Shi R, Zhang Y, Duan X, Li L, Zhang J, et al. A broadly neutralizing humanized ACE2-targeting antibody against SARS-CoV-2 variants. Nat Commun. 2021;12:5000. 10.1038/s41467-021-25331-x.
- Chen J, Li S, Lei Z, Tang Q, Mo L, Zhao X, et al. Inhibition of SARS-CoV-2 pseudovirus invasion by ACE2 protecting and Spike neutralizing peptides: An alternative approach to COVID19 prevention and therapy. Int J Biol Sci. 2021;17:2957-69. 10.7150/ijbs.61476.
Newsletter Issue 97, August 23, 2021:
- Surviving Covid-19 with Heparin? 8/4/21. Ten Cate H. N Engl J Med.
This editorial attempts to reconcile the differences in outcomes of the two studies below between critically ill and moderately ill COVID-19 patients who received heparin at therapeutic vs. thromboprophylactic doses. It is fairly clear that therapeutic anticoagulation does not provide increased benefit over thromboprophylaxis for critically ill patients; however, the degree of benefit of full anticoagulation over prophylaxis for patients with moderate disease remains an open question. - Therapeutic Anticoagulation with Heparin in Critically Ill Patients with Covid-19. 8/5/21. REMAP-CAP Investigators. N Engl J Med.
This randomized study of 1098 patients was stopped early because “In critically ill patients with COVID-19…therapeutic-dose anticoagulation with heparin did not result in a greater probability of survival to hospital discharge or a greater number of days free of cardiovascular or respiratory organ support than did usual-care pharmacologic thromboprophylaxis.” Major bleeding occurred in 3.8% of patients receiving therapeutic-dose anticoagulation vs. 2.3% receiving usual-care thromboprophylaxis. These data are the result of harmonized protocols of 3 international adaptive platform trials (REMAP-CAP, ACTIV-4A, and ATTACC). A limitation is that the majority of patients were in the UK where usual care changed from low-dose to intermediate dose prophylaxis during the study period, April-December 2020. - Therapeutic Anticoagulation with Heparin in Noncritically Ill Patients with Covid-19. 8/5/21. ATTACC Investigators. N Engl J Med.
This companion study reports outcomes following initial treatment with therapeutic vs. prophylactic heparin anticoagulation for 2,219 COVID-19 patients with moderate disease. Survival until hospital discharge without receipt of organ support during the first 21 days was 76.4% (801/1048) for those in usual-care thromboprophylaxis vs. 80.2% (939/1171) for those in the therapeutic anticoagulation group. Neither age, level of respiratory support at enrollment, nor thromboprophylaxis dose affected outcomes. The final posterior probability for superiority of therapeutic-dose anticoagulation vs. usual-care thromboprophylaxis was 97.3% in the high d-dimer cohort, 92.9% in the low d-dimer cohort, and 97.3% in the cohort with an unknown d-dimer level. A table summarizes secondary outcomes, including major bleeding in 1.9% receiving therapeutic dose vs. 0.9% receiving thromboprophylaxis.
Newsletter Issue 96, August 16, 2021:
- SAB Comment: The following two studies on vaccine-induced immune thrombocytopenia (VITT) provide two different views of a complex therapeutic question that remains unresolved. What is the appropriate dose (therapeutic versus prophylactic) and timing of anticoagulant therapy in the treatment of COVID-19 and what is the incidence of VITT in the general population stratified by age and sex following vaccination?
- Clinical Features of Vaccine-Induced Immune Thrombocytopenia and Thrombosis. 8/11/21. Pavord S. N Engl J Med.
A study of 294 patients presenting to UK hospitals (03/22-06/06 2021) found incidence of vaccine-induced immune thrombocytopenia and thrombosis (VITT) following ChAdOx1 nCoV-19 (AstraZeneca) vaccination among individuals younger than 50 years at least 1:50,000 which is consistent with previous reports. The study details diagnosis, patient demographics and common timeline for vaccination to symptomatology. Useful tables detail definition criteria (definite, probable, possible, unlikely) and clinicopathological findings. Age stratification notes incidence in older than 60 years at least 1/100,000. The authors conclude “The high mortality associated with VITT was highest among patients with a low platelet count and intracranial hemorrhage. Treatment remains uncertain, but identification of prognostic markers may help guide effective management.” - Cerebral venous thrombosis after vaccination against COVID-19 in the UK: a multicentre cohort study. 8/9/21. Perry RJ. Lancet.
Investigators studied 95 patients from 43 UK hospitals with image-confirmed cerebral venous thrombosis following vaccination for COVID-19 looking for vaccine-induced immune thrombotic thrombocytopenia (VITT), which was defined as acute thrombosis accompanied by D-dimer greater than 2,000 along with a minimum platelet count less than 150,000. Seventy-six (80%) of 95 patients were investigated for anti-PF4 antibodies, a reliable marker for VITT. Seventy in 96 had VITT, all following AstraZeneca vaccine. Of 26 without VITT, 21 had received AstraZeneca vaccines, and four had received Pfizer vaccines. VITT patients were younger (mean 47 vs. 57), more likely to have multiple venous thromboses (14% vs. 0) or hemorrhages (33% vs. 14%), and more disabled at discharge compared with non-VITT patients. Mortality was 29% in the VITT cohort vs. 4% in the non-VITT cohort. One non-VITT patient had serious extra-cerebral thrombosis. Non-heparin anticoagulant and intravenous immunoglobulin treatments were associated with an improved outcome. Diagnosis criteria are proposed.
- Clinical Features of Vaccine-Induced Immune Thrombocytopenia and Thrombosis. 8/11/21. Pavord S. N Engl J Med.
- Persistent Endotheliopathy in the Pathogenesis of Long COVID Syndrome. 8/10/21. Fogarty H. J Thromb Haemost.
Findings of pulmonary endotheliopathy and microvascular immunothrombosis have been highlighted in autopsies in acute COVID, but their contributions to Long-COVID are unknown. Long-COVID patients (n=50, age 50 + 17 years, medium post-COVID =68 days) showed that prothrombic markers (endogenous thrombin potential, peak thrombin, etc.) and endothelial activation markers (VWF:Ag, Factor VIII, etc.) and plasma soluble thrombomodulin were significantly elevated vs. controls (nonhospitalized asymptomatic, n=17, mean age 47 ± 12 years), especially in elderly, hospitalized and patients with co-morbidities. Typical acute phase markers (e.g., CRP, neutrophil counts, IL-6) were normal. Endotheliopathy assays (e.g., VWF) correlated inversely with the 6-Minute Walk Test.
Newsletter Issue 95, August 9, 2021:
- Covid-19 Breakthrough Infections in Vaccinated Health Care Workers. 7/28/21. Bergwerk M. N Engl J Med.
At the largest Israeli medical center, healthcare workers with COVID-19 exposure or symptoms underwent extensive evaluations from 1/20/21 – 4/28/21 to investigate infectivity and breakthrough infections. Breakthrough cases with neutralizing antibody (nAb) titers within a week before documented infection were matched with 4-5 uninfected controls. Among 1,497 healthcare workers fully vaccinated with BNT162b2 for whom RT-PCR data were available, 39 SARS-CoV-2 breakthrough infections were documented (0.4%). Eighty-five percent were B.1.1.7. (Alpha). Most were asymptomatic or mild, yet 19 had persistent symptoms at 6 weeks. nAb titers during the peri-infection period were lower in patients than in controls (ratio, 0.36). Higher nAb levels were associated with lower viral load. No secondary infections were documented. In all 37 patients for whom the suspected source of infection was identified, it was an unvaccinated person, mostly household members. - Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant. 7/21/21. Bernal JL. N Engl J Med.
British investigators used a test-negative case-control design to estimate the effectiveness of vaccination against symptomatic disease caused by the delta variant or the predominant alpha variant (B.1.1.7) over the period that the delta variant began circulating. With the Pfizer vaccine, the effectiveness of two doses was 93.7% among persons with the alpha variant (N=14,837) and 88.0% among those with the delta variant (N=4,272). Data ran up to 5/16/21. With the AstraZeneca vaccine, the effectiveness of two doses was 74.5% among persons with the alpha variant and 67.0% among those with the delta variant. Effectiveness was only 31% for alpha and 49% for delta after just one dose of either vaccine. - Immunogenicity and reactogenicity of heterologous ChAdOx1 nCoV-19/mRNA vaccination. 7/26/21. Schmidt T. Nature Med.
Heterologous priming with a single dose of the AstraZeneca ChAdOx1 nCoV-19 adeno vector vaccine followed by boosting with either the Pfizer or the Moderna mRNA vaccine is currently recommended in Germany. This study compares multiple aspects of immune response (spike-specific IgG, neutralizing antibodies, spike-specific CD4 T cells, and spike-specific CD8 T cell levels) in subjects receiving this heterologous regimen to the responses in subjects receiving two-dose homologous regimens with AstraZeneca vaccine or with an mRNA vaccine. All regimens were similarly well tolerated. Immune response levels were significantly higher with the heterologous regimens than after a two-dose AstraZeneca regimen and higher or comparable in magnitude to homologous mRNA vaccine regimens.
SAB Comment: Heterologous vaccine strategies were initially pioneered in HIV and Ebola. Currently, at least 5 EU countries have recommended it as a means of producing fewer side effects than a two-dose AstraZeneca regimen. - Efficacy and safety of remdesivir in hospitalised COVID-19 patients: a systematic review and meta-analysis. 7/31/21. Angamo MT. Infection.
This is a meticulous review of pooled data taken from 4 RCTs and 3 controlled observational trials covering a 12-month span starting December 2019 comparing remdesivir treatment to placebo or standard care. Remdesivir significantly accelerated recovery at day 7 (21%) and day 14 (29%), lowered the incidence of high oxygen flow therapy by 27% and mechanical ventilation by 47%, and decreased mortality on day 14 by 39% but not on day 28. Serious adverse effects were less common in the remdesivir group and the authors conclude that remdesivir treatment is effective and safe early in SARS-CoV-2 infections. - Dexamethasone and tocilizumab treatment considerably reduces the value of C-reactive protein and procalcitonin to detect secondary bacterial infections in COVID-19 patients. 8/6/21. Kooistra EJ. Crit Care.
This prospective observational study from a single Dutch medical center compares the established predictive value of inflammatory biomarkers C-reactive protein (CRP) and procalcitonin (PCT) in identifying secondary bacterial infections in severe COVID-19 patients admitted between March and April 2020. A second patient cohort was treated in their ICU after August 2020 which received dexamethasone with or without a single dose of tocilizumab 8mg/kg IV. Results showed marked blunting of the CRP and PCT response during and a rebound after cessation of immunosuppression which could be falsely interpreted as a signal of secondary infection, while the blunted response may mask ongoing secondary infection. - Endothelium-associated biomarkers mid-regional proadrenomedullin and C-terminal proendothelin-1 have good ability to predict 28-day mortality in critically ill patients with SARS-CoV-2 pneumonia: A prospective cohort study. 8/3/21. van Oers JAH. J Crit Care.
This observational cohort study assessed baseline levels of two inflammatory markers, midregional proadrenomedullin (MR-proADM) and C-terminal proendothelin-1 (CT-proET-1) as predictors of 28-day mortality in 105 critically ill COVID-19 pneumonia patients. The area under the curve for prediction of 28-day mortality for MR-proADM and CT-proET-1 were 0.84 and 0.79 respectively. An MR-proADM level of d≥1.57 nmol/L or a CT-proET-1 level of ≥ 111 pmol/L at baseline were significant predictors for 28-day mortality (HR 6.80 and HR 3.72 respectively) and were significantly better predictors than other, more common, inflammatory markers. - Drug-induced phospholipidosis confounds drug repurposing for SARS-CoV-2. 7/30/21. Tummino TA. Science.
Many drugs are reported to have in vitro activity against SARS-Co-V-2. Some of these “repurposed” drugs including hydroxychloroquine, azithromycin and amiodarone are already in trials. This investigation discovered a shared mechanism of many “repurposed” drugs: phospholipidosis, which is a phospholipid storage disorder induced by cationic amphiphilic drugs. For all 23 drugs tested, development of intracellular phospholipidosis correlated with antiviral “efficacy.” Conversely, drugs active against the same targets that did not induce phospholipidosis were not antiviral. Phospholipidosis does not reflect specific target-based activities, but is a toxic confound. Early detection of phospholipidosis could eliminate screening artifacts, steering focus on molecules with real potential. The accompanying editorial points out that that “mechanism-informed” strategy for drug repurposing can work (e.g., remdesivir) and may result in clinically useful results. Conversely, repurposing drugs based on hypothesis-free cellular screens “has not yet yielded any effective treatments for COVID-19, nor for any disease.” These latter mass screenings are not shortcuts, but rather costly, scientific “dead-ends.”
Newsletter Issue 94, July 26, 2021:
- Masitinib is a broad coronavirus 3CL inhibitor that blocks replication of SARS-CoV-2. 7/21/21. Drayman N. Science.
Out of 1,900 clinically tested drugs tested in vitro, eight drugs inhibited the activity of one of two SARS-CoV-2’s major proteases, 3CLpro. The most potent inhibitor was masitinib, an orally bioavailable tyrosine kinase inhibitor (TKI) previously tested in human cancers, neurologic and mast cell disorders. In SARS-CoV-2, masitinib acts as a competitive inhibitor of 3CLpro, not as a TKI. SARS-CoV-2-infected mice treated with masitinib showed >200-fold viral titer reductions in nose and lung with reduced lung inflammation. Masitinib was effective in vitro against alpha, beta, and gamma variants. Clinical trials are recommended with use of masitinib early in infection.
SAB Comment: Clinical studies of masitinib are currently on temporary hold due to the potential for adverse cardiac effects. - Physical Therapy and Sedation While on Extracorporeal Membrane Oxygenation for COVID-19-Associated Acute Respiratory Distress Syndrome. 7/21/21. Bohman JK. J Cardiothorac Vasc Anesth.
This is a study of matched cohort patients on veno-venous ECMO for COVID and non-COVID ARDS, comparing sedative requirements and ability of patients to participate in physical therapy (PT). Despite earlier suggestions that COVID-19 patients required higher sedative requirements, in this study at two Mayo Clinic sites (Rochester and Jacksonville), sedative requirements, RASS scores and PT participation were similar with no statistical differences between COVID-19 and non-COVID-19 ECMO patients. - Performance Analysis of the National Early Warning Score and Modified Early Warning Score in the Adaptive COVID-19 Treatment Trial Cohort. 7/19/21. Colombo CJ. Crit Care Explor.
This study demonstrated moderate prognostic performance of predictive scores in patients with severe coronavirus disease and validates the addition of age as a predictor in severe COVID-19 disease. Predictive scores remain an aid to clinician’s experience in decision making and add value under resource constraints.
Newsletter Issue 93, July 19, 2021:
- Baricitinib reduces 30-day mortality in older adults with moderate-to-severe COVID-19 pneumonia. 7/8/21. Abizanda P. J Am Geriatr Soc.
This retrospective, propensity matched cohort study from Spain compared the efficacy of baricitinib in 2 groups of hospitalized patients aged 70 and older (mean age 79) and younger than 70 years (mean age 59), to matched groups of patients receiving standard therapy alone. There was an 18.5% absolute mortality risk reduction in patients older than 70 years and a lower 30-day adjusted fatality rate (HR 0.21; 95% CI 0.09–0.47; p < 0.001). Standard therapy included lopinavir/ritonavir, hydroxychloroquine and corticosteroids in the majority of patients. The result of this study corroborates the evidence presented in the ACTT-2 and the COV-BARRIER trials and other clinical studies. - Association Between Administration of IL-6 Antagonists and Mortality Among Patients Hospitalized for COVID-19: A Meta-analysis. 7/6/21. WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. JAMA.
This meta-analysis analyzed IL-6 antagonists vs. usual care including corticosteroids or placebo and 28-day all-cause mortality and other outcomes included nearly 11,000 patients from 27 clinical trials. Twenty-eight-day mortality was 1407/6449 randomized to IL-6 antagonists vs. 1158/4481 randomized to usual care or placebo; OR, 0.86; P = .003, and OR 0.72 for those also receiving corticosteroids. Absolute mortality was 22% for IL-6 antagonists vs. an assumed mortality of 25% for usual care or placebo. The ORs for progression to invasive mechanical ventilation or death vs. usual care or placebo was 0.77 for all IL-6 antagonists. A well-written editorial discusses the study’s findings, significance, strengths, and weaknesses. - Standard prophylactic versus intermediate dose enoxaparin in adults with severe COVID-19: a multi-center, open-label, randomized controlled trial. 7/8/21. Perepu US. J Thromb Haemost.
One hundred seventy-six hospitalized patients with COVID-19 who either were in the ICU (n=107) or had coagulopathy were randomized to prophylactic or intermediate dose enoxaparin. Thirty-day mortality, bleeding and rates of thrombosis were not different between the two arms. These data help the clinician with the treatment of COVID-19 patients with respect to prophylaxis for deep venous thrombosis and support prior data.
SAB Comment: This article confirms the prior data from Iran’s Inspiration trial that was previously reviewed by the SAB. - U shape association of hemoglobin level with in-hospital mortality for COVID-19 patients. 7/2/21. Kuno T. J Thromb Thrombolysis.
This article examines the data from 9467 hospitalized COVID-19 patients and found that the mortality rate is not only associated with anemia (hemoglobin < 10 g/dL) but also higher hemoglobin levels (hemoglobin >16 g/dL). While this finding is not novel outside of COVID-19, this extensive dataset from a large hospital system in New York City adds to the literature. The paper is concise and offers new avenues for further investigation. - Implementation and Outcomes of a Mobile Extracorporeal Membrane Oxygenation Program in the United States During the Coronavirus Disease 2019 Pandemic. 6/28/21. Odish MF. J Cardiothorac Vasc Anesth.
This article examines the use of mobile ECMO for patients being transferred in Southern California in 21 patients with COVID-19. For mobile ECMO, a cannulation team travels to the patient’s hospital with all of the necessary equipment to start the ECMO. They accompany the patient back to accepting tertiary care center for continuing care. The paper adds to the literature and offers specific practical tips for the clinician. It focuses on lessons learned and pitfalls to be avoided. It is well written and engaging.
Newsletter Issue 92, July 12, 2021:
- Risk factors for long covid in previously hospitalised children using the ISARIC Global follow-up protocol: A prospective cohort study. 7/2/21. Osmanov IM. Eur Respir J.
This is a study of 518 COVID-19 infected children admitted to a pediatric hospital in Moscow and followed for 5-12 months — the largest follow-up pediatric study to date. Parents were interviewed using an internationally designed and accepted protocol. Average age was 10.4 years (<1-18 years range) and near equal distribution between sexes. Long COVID was found in 24.3% of children. Fatigue and sleep disturbance were the most common complaints followed by loss of smell. Symptoms declined over time. Risk factors for persistent symptoms were patients older than 6 years old and a history of allergic disease. Psycho-social issues were uncommon and no deaths were reported. - Efficacy and safety of remdesivir in COVID-19 caused by SARS-CoV-2: a systematic review and meta-analysis. 6/25/21. Singh S. BMJ Open.
Investigators reviewed and analyzed 4/52 RCTs with a total of 7324 patients to evaluate the efficacy of remdesivir for COVID-19 patients. The results indicated that there is no benefit with mortality rate. A benefit favoring remdesivir over control does exist in terms of rates of clinical improvement and faster time to clinical improvement. No difference was shown in respiratory failure in two (flawed) studies. All outcomes except mortality were influenced by two studies which were riddled with high risk of bias and low quality evidence. In a cost to benefit analysis, remdesivir has a limited role in poor countries.
SPECIAL EDITION: Newsletter Issue 91, July 7, 2021:
- Variants of concern are overrepresented among post-vaccination breakthrough infections of SARS-CoV-2 in Washington State. 6/24/21. McEwen AE. Clin Infect Dis.
In an effort to determine mRNA vaccine efficacy against SARS-CoV-2 variants of concern (VOC), the University of Washington performed genetic sequencing of the SARS-CoV-2 virus on all positive PCR samples between February 23 and April 27, 2021. Of the 5,174 unvaccinated cases, 68% were VOC compared to 100% of the 20 breakthrough cases in vaccinated patients. Most breakthrough cases were symptomatic (~80%) but none were hospitalized. No single VOC was significantly more common in the breakthrough cases compared with unvaccinated cases. This is consistent with previous reports that mRNA vaccines provide excellent protection to all current strains of the virus, though there is a rare VOC breakthrough.
SAB Comment: As the pandemic continues, more VOC that could be a problem even for vaccinated people may evolve. This emphasizes the importance of the current vaccination effort and world-wide control of the pandemic. - The SARS-CoV-2 mRNA vaccine breakthrough infection phenotype includes significant symptoms, live virus shedding, and viral genetic diversity. 6/12/21. Pollett SD. Clin Infect Dis.
This pilot report from the US Military Health System examined 24 PCR confirmed infections more than 14 days after full Pfizer (92%) and Moderna (8%) vaccination. Sixty-seven percent had no co-morbidities, 63% were health care workers, and 71% were White. Five were asymptomatic, and none required hospitalization; however, symptoms lasted up to 2 weeks and were reported as severe in 3. Viral cultures and complete genomic sequencing were performed in many cases. Strains included wild type as well as variants of concern. Some were shedding live virus 7 days after symptom onset. Authors recommend larger, prospective studies of vaccine breakthrough infections.
SAB Comment: The CDC recently reported 4,115 cases from 47 states of breakthrough infections in fully vaccinated individuals who were hospitalized or died (mortality 18%) as of 6/21/21. Seventy-six percent were older than 65 years. Twenty-six percent of hospital admissions were not initiated for COVID-19. One hundred forty-two in 750 fatalities (19%) were not attributed to COVID-19. “The number of COVID-19 vaccine breakthrough infections reported to CDC likely are an undercount of all SARS-CoV-2 infections among fully vaccinated persons. National surveillance relies on passive and voluntary reporting, and data might not be complete or representative.” - Three Doses of an mRNA Covid-19 Vaccine in Solid-Organ Transplant Recipients. 6/23/21. Kamar N. N Engl J Med.
This letter documents the humoral antibody response to 3 doses of the Pfizer-BioNTec vaccine in 101 solid organ transplant recipients 97 months post transplant, none of whom have become infected. The second dose was given 30 days after the first, and the third, 60 days after the second. Titers for spike protein antibodies were obtained before the first, second and third doses and one month after the third dose. Before the second dose, only 4 patients had antibodies, increasing to 40% before the third dose. After the third dose, 68% had antibodies. 33 patients (who were older, with a higher degree of immunosuppressive and a lower GFR) presumably remained at risk for infection.
SAB Comment: Besides antibodies, the immune system has redundant lines of defense including T-cells (e.g., cellular immunity) that may be protective though not easily assessed. We await further “real world” studies on actual numbers and severity of infections in solid organ transplant patients, regardless of antibody levels. - Mortality after surgery with SARS-CoV-2 infection in England: a population-wide epidemiological study. 6/21/21. Abbott TEF. IBr J Anaesth.
This retrospective British NHS database study addresses surgical mortality associated with SARS-CoV-2 from 1/1/2020 to 2/28/2021. Of 2.5 million surgeries, 1.0% of patients died and 1.1% of patients were infected. The mortality was 21% in patients with SARS-CoV-2 and 0.8% in those uninfected (OR 5.7). With elective surgery, 1% were infected, and mortality was 7.1%, compared to 0.1% (OR 25.8). Emergency procedure mortalities were 25.1% compared to 3.4% (OR 5.5). Statistics include data for procedure types and disease severity, and demonstrate the safety of elective procedures, with precautions, in healthy patients with no SARS-CoV-2 history. The authors estimate about one-half of 4.5 million expected surgical procedures were postponed.
Newsletter Issue 90, June 28, 2021:
- Tofacitinib in Patients Hospitalized with Covid-19 Pneumonia. 6/16/21. Guimarães PO. N Engl J Med.
This randomized, double-blind, placebo-controlled and industry-sponsored trial (“STOP COVID”) involving 289 hospitalized patients with Covid-19 pneumonia in Brazil showed tofacitinib superior to placebo in reducing the incidence of death or respiratory failure (18 vs 29% – HR 0.63). Overall mortality was 2.8% in the tofacitinib group vs. 5.5% for placebo. Standard therapy (antivirals, glucocorticoids, anticoagulation) was comparable between groups, as were adverse events. This study corroborates the findings of the NIH funded ACTT-2 trial and the value of JAK inhibition for the treatment of Covid-19 pneumonia in patients who are not yet receiving invasive mechanical ventilation.
SAB Comment: NIH COVID-19 treatment guidelines recommend against the use of JAK inhibitors other than baricitinib for the treatment of COVID-19, except in a clinical trial. - Underlying Medical Conditions Associated With Severe COVID-19 Illness Among Children. 6/7/21. Kompaniyets L. JAMA Netw Open.
A data rich CDC review studying 43,465 children 18 years old and younger hospitalized with COVID-19 infection through January 2021. After a complete description on data retrieval and analysis, results revealed children with diabetes, obesity and those with cardiac anomalies were more commonly hospitalized than previously healthy children. Overall children with any chronic disease were hospitalized 3 times more frequently. Asthma was a risk for severe infection. Children younger than 2 years old and born prematurely were prone to hospitalization. Finally, Hispanic and Black children suffered severe infection more frequently than Whites. - Awake prone positioning in patients with hypoxemic respiratory failure due to COVID-19: the PROFLO multicenter randomized clinical trial. 6/15/21. Rosén J. Crit Care.
In this small, randomized, controlled study from Sweden, the efficacy of awake prone positioning (APP) was evaluated in 75 patients with COVID-19 in moderate to severe respiratory failure. Compared with standard care, implementation of a 16 hour/day protocol for APP increased the duration of prone positioning but did not affect the rate of intubation. The study was halted early due to futility. When secondary outcomes were analyzed, the only difference between groups was a reduction of pressure sores in the APP group.
SAB Comment: Though small, this is a well-designed prospective and randomized trial of APP in COVID-19 patients and confirms retrospective studies that question the efficacy of APP. - Taskforce report on the diagnosis and clinical management of COVID-19 associated pulmonary aspergillosis. 6/23/21. Verweij PE. Intensive Care Med.
This is a thorough and lengthy review by an international group of 28 experts prompted by the relatively high incidence of COVID-associated pulmonary aspergillosis (CAPA) seen in severely ill COVID-19 patients. The prevalence of CAPA varied between 0 and 33%. Bronchoscopy and bronchoalveolar lavage (BAL) remain the cornerstone of CAPA diagnosis. Most patients diagnosed with CAPA lack traditional host factors, but pre-existing structural lung disease and immunomodulating therapy may predispose to CAPA risk. Computed tomography seems to be of limited value to rule CAPA in or out, and serum biomarkers are negative in 85% of patients. As the mortality of CAPA is around 50%, antifungal therapy is recommended for BAL-positive patients, while the authors recommend against routinely stopping concomitant corticosteroid or IL-6 blocking therapy in CAPA patients.
Newsletter Issue 89, June 21, 2021:
- Further Evidence Supporting the Use of Prophylactic Anticoagulation in Hospitalized Patients With COVID-19. 6/11/21. Dicks AB. JAMA Netw Open.
This editorial provides a succinct review of current understanding regarding anticoagulation for COVID-19 patients. It accompanies a retrospective report in the same journal that looked at 60-day mortality in addition to in-hospital outcomes for patients treated with either prophylactic or therapeutic anticoagulation during the first wave in the US. Although randomized controlled studies are still lacking, evidence suggests that only prophylactic anticoagulation is associated with reduced 60-day mortality. (Adjusted hazard ratio 0.71 for prophylactic vs 0.92 for therapeutic dosing). - Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION): an open-label, multicentre, randomised, controlled trial. 6/7/21. Lopes RD. Lancet.
This trial from Brazil, randomized, hospitalized patients with COVID-19 between either therapeutic anticoagulation or prophylaxis. The therapeutic arm was oral rivaroxaban (20 mg or 15 mg daily) for stable patients (94%), or initial subcutaneous enoxaparin (1 mg/kg twice per day) or intravenous unfractionated heparin for clinically unstable patients, followed by rivaroxaban to day 30. Prophylactic anticoagulation was standard in-hospital enoxaparin or unfractionated heparin. No difference was found in the mortality between the two arms with increased bleeding in the therapeutic arm. This article suggests that the use of therapeutic-dose rivaroxaban should be avoided in these patients in the absence of an evidence-based indication for oral anticoagulation. - Association between previous anticoagulant use and mortality among hospitalized patients with COVID-19. 6/3/21. Gülcü O. J Thromb Thrombolysis.
This article from Turkey reviews 5575 patients who were hospitalized with COVID-19. They examined the use of prehospitalization anticoagulation (n= 451) with direct oral anticoagulants (n=383) or warfarin (n=68). After propensity scoring, this study found that previous use of oral anticoagulation was associated with lower mortality in patients hospitalized due to COVID-19 (adjusted hazard ratio 0.62, p=0.030). These data add to the conflicting data examining prehospital anticoagulation in COVID-19.
Newsletter Issue 88, June 14, 2021:
- The year in review: mechanical ventilation during the first year of the COVID-19 pandemic. 5/7/21. Kallet R. Respiratory Care.
This thoughtful, well-written, and thorough narrative review of COVID-19 ARDS (C-ARDS) includes 201 references and covers the evolution of best respiratory care practices to date. The overarching question is whether C-ARDS is significantly different from ARDS. Longstanding debates regarding phenotypes and taxonomy are discussed. The evolution of C-ARDS management and physiologic evidence for respiratory care are presented. Topics include phenotypic differences, mechanisms of hypoxia, noninvasive ventilation, timing of intubation, ventilation practices, PEEP, pathologic and radiologic findings, self-inflicted lung injury, lung mechanics, and cross infection. The author concludes that from a respiratory management perspective, C-ARDS differs little from ARDS of other etiologies. - Closed-Loop Versus Conventional Mechanical Ventilation in COVID-19 ARDS. 6/8/21. Wendel Garcia PD. J Intensive Care Med.
Closed-Loop (C-Loop) is an automated/autopilot ventilation mode which integrates key patient respiratory parameters into automatic ventilator adjustments that provide a high degree of lung protective ventilation (LPV) and result in a reduced frequency of hypoxemic episodes. This randomized, prospective study compares ventilator support for COVID-19 ARDS patients using either C-Loop (n= 23) or conventional mechanical ventilation (Con-V, n= 17). The C-Loop group showed a statistically significant improvement in the dynamic mechanical power necessary, higher total lung compliance and PF ratio and lowered VD/ VT, PEEP, and Fio2 while maintaining adequate PaO2. This suggests that C-Loop ventilation may decrease the risk of ventilator induced lung injury while reducing the number of necessary human ventilator adjustments. The paper describes an impressive tool with a convincing radar graph for its practical utility but provides limited outcome data.
SAB Comment: This is a small, futuristic, innovative, and intriguing pilot study of the feasibility of an automated ventilator-adjustment device to better provide lung protective ventilation. - Rehabilitation post-COVID-19: cross-sectional observations using the Stanford Hall remote assessment tool. 5/27/21. O’Sullivan O. BMJ Mil Health.
These authors report the development and use (April to Nov 2020) of a video teleconferencing tool to evaluate rehabilitation needs for patients with ongoing post-COVID-19 symptoms and included patients with COVID syndromes who never had a confirmatory COVID-19 viral test. They found that the initial severity of symptoms did not predict the level of ongoing disability. They conclude that post-COVID-19 symptoms should be considered in all patients, regardless of the acute illness severity and whether they have had laboratory confirmation. They find that a significant proportion of patients require assessment and management, with symptoms such as shortness of breath, fatigue, and mood disorders impacting activities of daily living and return to work. - Hospitalization of Adolescents Aged 12-17 Years with Laboratory-Confirmed COVID-19 – COVID-NET, 14 States, March 1, 2020-April 24, 2021. 6/10/21. Havers FP. MMWR Morb Mortal Wkly Rep.
In the US, “Most COVID-19-associated hospitalizations occur in adults, but severe disease occurs in all age groups, including adolescents aged 12–17 years. COVID-19 adolescent hospitalization rates from COVID-NET peaked at 2.1 per 100,000 in early January 2021, declined to 0.6 in mid-March, and rose to 1.3 in April. Among hospitalized adolescents, nearly one third required intensive care unit admission, and 5% required invasive mechanical ventilation; no associated deaths occurred. Recent increased hospitalization rates in spring 2021 and potential for severe disease reinforce the importance of continued COVID-19 prevention measures, including vaccination and correct and consistent mask wearing among persons not fully vaccinated or when required.” –MMWR Summary
Newsletter Issue 87, June 7, 2021:
- COVID-19 Vaccine Breakthrough Infections Reported to CDC – United States, January 1-April 30, 2021. 5/27/21. CDC COVID-19 Vaccine Breakthrough Case Investigations Team. MMWR Morb Mortal Wkly Rep.
In this brief weekly report, the CDC provides an important glimpse into the post-vaccination scenario in the US. During the 4 months ending April 30, 2021, a total of 10,262 breakthrough infections have been reported. Of those, 63% were female, median age 58 years, of which 27% were asymptomatic, 10% hospitalized and 2% died. By the end of the study, although 100 million individuals had been vaccinated in the US, SARS-CoV-2 transmission was still in full swing with 355,000 new cases daily. Variants were detected at a similar rate among vaccinated and non-vaccinated patients. Underreporting of asymptomatic cases and limited RNA sequencing represent current and future limitations to these statistics. Notably, beginning May 1, 2021, the CDC transitioned from monitoring all reported COVID-19 vaccine breakthrough infections to investigating only those among patients who are hospitalized. Monthly reports and additional information on vaccination breakthrough initiatives by the CDC can be found here. - Safety, Immunogenicity, and Efficacy of the BNT162b2 Covid-19 Vaccine in Adolescents. 5/27/21. Frenck RW Jr. N Engl J Med.
The BNT162b2 COVID-19 RNA vaccine (Manufacturer: Pfizer, Inc., and BioNTech) was proven effective with few side effects in 12-15 year old recipients (n=1131) who received 2 injections 21 days apart versus controls (n=1129). Among participants without evidence of previous SARS-CoV-2 infection, no COVID-19 cases with an onset of 7 or more days after dose 2 were noted among BNT162b2 recipients, and 16 cases occurred among placebo recipients. The observed vaccine efficacy was 100% (95% CI, 75.3 to 100). - The characteristics and outcomes of critically Ill patients with COVID-19 who received systemic thrombolysis for presumed pulmonary embolism: an observational study. 5/9/21. So M. J Thromb Thrombolysis.
This article reviews the clinical outcome of the use of systemic tissue plasminogen activator (tPA) for suspected pulmonary embolism (PE) in 57 critically ill COVID-19 patients from 5 hospitals in NYC during March and April 2020. All of the patients were suspected to have pulmonary embolization based upon echocardiography (16%) or clinical findings but were too unstable to have CT confirmation. Forty-nine percent demonstrated short-term improvement with tPA. However, 89% died in the hospital.
SAB Comment: This study is the largest cohort reported that we have seen for this problem, and demonstrates the poor outcomes of patients suspected of PE with or without tPA. - Implications of early respiratory support strategies on disease progression in critical COVID-19: a matched subanalysis of the prospective RISC-19-ICU cohort. 5/26/21. Wendel Garcia PD. Crit Care.
Propensity matching was performed on an initial group of 1,421 COVID-19 ARDS patients from the large European RISC-19-ICU cohort resulting in propensity matched patients in cohorts treated initially in the ICU with standard O2 therapy (SOT) (n=85), high-flow oxygen therapy (HFNC) (n=87), non-invasive ventilation (NIV) (n=87) and invasive mechanical ventilation (IMV) (n=92). The ICU intubation rate was lower in patients initially supported with HFNC and NIV compared to those who received SOT. Compared to the other respiratory support strategies, NIV was associated with a higher overall ICU mortality (SOT: 18%, HFNC: 20%, NIV: 37%, IMV: 25%, p = 0.016). The authors recommend a closely observed trial of HFNC for ICU patients not immediately requiring IMV.
SAB Comment: This retrospective analysis may best be thought of as a recommendation to perform an RCT to support or challenge these conclusions.
Newsletter Issue 86, June 2, 2021:
- Immunogenicity of COVID-19 mRNA Vaccines in Pregnant and Lactating Women. 5/13/21. Collier AY. JAMA.
This excellent cohort report of the immunologic response to mRNA vaccination in pregnant (30), lactating (16), nonpregnant (57), and post SARS-CoV-2 pregnant (22) and nonpregnant women (6) demonstrates its effectiveness in pregnancy and potential for newborn protection. At 2 to 8 weeks post second dose, both neutralizing antibodies and cellular responses were measured in maternal serum, cord blood, and breast milk. Vaccination-produced neutralizing antibody titers higher than those from infection. Antibodies were detected in both cord blood and breast milk. Neutralizing antibody titers to variants were reduced, but cellular responses were preserved. A very useful glossary of immunological assays is included. - Coronavirus Disease 2019-Associated PICU Admissions: A Report From the Society of Critical Care Medicine Discovery Network Viral Infection and Respiratory Illness Universal Study Registry. 5/9/21. Tripathi S. Pediatr Crit Care Med.
A country-wide study from the Society of Critical Care Medicine reporting on COVID-19 infected children admitted to intensive care over an 11-month period with and without associated MIS-C. Age, gender and race were similar between groups. The low numbers enrolled provide evidence that the disease incidence of either variety is low in children. MIS-C resulted in longer-lasting severe illness, but the overall mortality of 3.8% was low and similar between groups.
Newsletter Issue 85, May 24, 2021:
- Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised controlled, open-label, platform trial. 5/17/21. RECOVERY Collaborative Group. Lancet.
In this randomized open-label study, 5795 hospitalized patients received high-titer convalescent plasma (CP) plus usual care and 5763 received usual care alone in 177 UK National Health Service hospitals. Ninety-two percent received corticosteroids. The study was halted prematurely, as there were no significant differences between groups in all-cause 28-day mortality (24%), progression to invasive ventilation (12-13%), renal replacement therapy (4%) or the proportion discharged from hospital within 28 days (66%). Mean age was 63, nearly 2/3 were male, and 77% were white. Median number of days since symptom onset was 9. Only 5% required mechanical ventilation at randomization. A well-written editorial reviewing this study and findings of other studies of CP can be found here.
SAB Comment: The RECOVERY trial includes the largest randomized study thus far of CP therapy for COVID-19. Although some retrospective observational studies of CP were encouraging, randomized controlled studies have not confirmed benefit. Questions remain about whether the average timing of CP therapy in this study was beyond the window of potential efficacy and whether selected patients may benefit from CP, particularly those with immune deficiencies. - A SARS-CoV-2 neutralizing antibody with extensive Spike binding coverage and modified for optimal therapeutic outcomes. 5/11/21. Guo Y. Nature.
The current monoclonal antibodies (mAb) from Regeneron and Lilly are based on the Hunan strain Spike sequence present prior to the emergence of mutants. Chinese scientists now report the development of a mAb called P4A1 that inhibits the Spike Receptor Binding Motif of the Spike Receptor-Binding Domain and acts against wild type and mutant Spike proteins. Also, P4A1 was engineered for safety, to extend its half-life and to reduce risk for Antibody-Dependent Enhancement of infection. In a rhesus monkey COVID model, a single infusion resulted in complete viral clearance. These data suggest P4A1’s potential against SARS-CoV-2 related diseases. - Non-steroidal anti-inflammatory drug use and outcomes of COVID-19 in the ISARIC Clinical Characterisation Protocol UK cohort: a matched, prospective cohort study. 5/17/21. Drake TM. Lancet Rheumatol.
This prospective, multicenter cohort study shows convincingly that patients who take NSAIDs before and in the early stages of a SARS-CoV-2 infection are not at a higher risk of dying or experiencing more severe disease. Using a proven data mining protocol, 72,179 hospitalized patients in 255 hospitals in the UK, with confirmed COVID-19, were enrolled and analyzed. Of those patients, 4,211 or 5.8% used NSAIDS (but not aspirin) before their illness. Propensity score matching resulted in balanced, well matched treatment groups and matched odds ratios for mortality, ICU admission, invasive ventilation, acute kidney injury, among others, showed no statistical difference. The authors urge policy makers to review advice issued early in the course of the pandemic regarding the use of NSAIDs and disease severity.
Newsletter Issue 84, May 17, 2021:
- Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. 5/8/21. Haas EJ. Lancet.
This is a prospective, longitudinal cohort study of 83 severe COVID-19 patients (admitted February and March). This data-rich study demonstrates graphically and convincingly the effectiveness of 2 doses of the Pfizer-BioNTech vaccine against a range of SARS-CoV-2 outcomes in Israel using surveillance data from the first 4 months of the vaccination campaign which began in December 2020. By April 3, 2021, 72% of 6.5 million people over age 16 had been vaccinated and the incidence rate dropped from 91.5 in unvaccinated individuals to 3.1 per 100,000 person-days in those fully vaccinated. Effectiveness against critical illness and death was 97.5% and 96.7% respectively. Widespread testing revealed effectiveness of the vaccine against the predominant B.1.1.7. (British) variant. Aspects of the Israeli health care system, concomitant lockdown measures as well as cultural and ethnic influences vis-à-vis the goal of achieving herd immunity are discussed. The Israeli Ministry of Health and Pfizer collaborated on this project. - Effectiveness of the BNT162b2 Covid-19 Vaccine against the B.1.1.7 and B.1.351 Variants. 5/5/21. Abu-Raddad LJ. New Engl J.
This letter to the editor reports effectiveness of the Pfizer-BioNTech vaccine against UK and S. African variants that represented 50% and 44.5% of infections, respectively, in the Qatari research cohort community at the time of study. Estimated vaccine effectiveness against any documented B.1.1.7 variant infection was 89.5% (95% CI 85.9-92.3) and 75% against B.1.351 (CI (70.5–78.9) at 14 or more days after second doses in nearly 400,000 people. Effectiveness against severe, or fatal disease due to any SARS-CoV-2 variant was 97.4% (95% CI, 92.2-99.5). Although effectiveness against the B.1.351 variant was ~20% below previous reports from the clinical trial or real-world conditions in Israel and the US, protection from hospitalization or death was >90%. Effectiveness was found to be significantly improved after second dose. - SARS-CoV-2 vaccine and thrombosis: Expert opinions. 5/4/21. Elalamy I. Thromb Haemost.
This article reviews the status of 4 COVID-19 vaccines (Pfizer, Moderna, Johnson & Johnson and AstraZeneca) with respect to thrombosis from an international viewpoint. Not only is it current and timely, but reviews:- What is known about the pathophysiology;
- Goes over the risk/benefit ratio of vaccination;
- What to do if there is a concern for thrombosis after vaccination; and
- What not to do.
Overall, this comprehensive article focuses on clinically relevant issues in a concrete fashion.
- Association of Maternal SARS-CoV-2 Infection in Pregnancy With Neonatal Outcomes. 4/29/21. Norman M. JAMA.
To determine the outcome in newborn infants of mothers testing positive for SARS-CoV-2 in pregnancy, this prospective cohort study looked at the outcomes of 88,159 infants born in Sweden during the first 10 months of the pandemic. After matching infants by maternal characteristics, the 2,323 infants of SARS-CoV-2-positive mothers were found to have more respiratory problems (2.8% vs 2.0%, OR 1.42), mostly explained by a more preterm birth. Mortality, breastfeeding rates at discharge, length of stay in neonatal care, hypoxic-ischemic encephalopathy, meconium aspiration, pneumonia, sepsis, and hypoglycemia did not differ significantly between the two groups. Twenty-one (0.9%) of the 2,323 infants of SARS-CoV-2-positive mothers had positive PCR tests, most with no morbidity and none with pneumonia. View a pertinent accompanying editorial here.
Newsletter Issue 83, May 10, 2021:
- 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: a prospective study. 5/8/21. Wu X. Lancet Respir Med.
This is a prospective, longitudinal cohort study from China of 83 severe COVID-19 patients (admitted February and March 2020, so none received glucocorticoids) who did not require IMV, yet still had 29-day hospital stays. Patients with HTN, DM, CVD, cancer, any pulmonary disease or tobacco use had been excluded. At 3-, 6-, 9- and 12-months post admission, they underwent pulmonary function testing, documenting abnormalities with gradual improvement even after 9 months. Radiological abnormalities (24%) and DLCO less than 80% of predicted (33%) persisted at 12 months despite near normal lung volumes, 6M walk and dyspnea assessment.
SAB Comment: These results indicate that even previously healthy patients who have recovered from COVID-19 may warrant pulmonary evaluation and consideration of timing regarding elective surgery. - Mortality after In-Hospital Cardiac Arrest in Patients with COVID-19: A Systematic Review and Meta-Analysis. 5/8/21. Ippolito M. Resuscitation.
This is a well-performed meta-analysis of resuscitation (CPR) following in-hospital cardiac arrest, confirming bleak survival statistics. The article includes an interesting debate regarding universal do not resuscitate orders for COVID-19 arrest resuscitation and comparison with ICU resuscitation of comparably ill patients without COVID-19. The authors suggest further discussion and data analysis is necessary following improved results for in-hospital cardiac arrest (IHCA) over time. Conclusion: Although one of three COVID-19 patients undergoing IHCA may achieve return of spontaneous circulation, 90% are not expected to survive 30 days or to hospital discharge. - Trends in Patient Characteristics and COVID-19 In-Hospital Mortality in the United States During the COVID-19 Pandemic. 5/3/21. Roth GA. JAMA Netw Open.
This analysis of mortality trends in the US among 20,736 patients in 107 hospitals in 31 states comes from the American Heart Association COVID-19 cardiovascular disease registry. In comparison with March/April patients, the odds ratio of mortality decreased approximately one-third later in the year, after adjusting for age, sex, medical history, and COVID-19 severity. ICU length of stay, use of mechanical ventilation, and mortality in age groups over 50 decreased, although mortality remained highly associated with age. Use of corticosteroids and remdesivir increased. Reasons and other independent risk factors are discussed. - Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. 5/2/21. RECOVERY Collaborative Group. Lancet.
This long-awaited trial assessing the effectiveness of tocilizumab differentiated itself from several earlier attempts in two important aspects:- It enrolled 4,116 of 21,550 adults at 131 sites in the UK as part of the RECOVERY trial between April 23, 2020 and January 24, 2021 and is therefore adequately powered and statistically sound.
- It demonstrated a small but significant benefit across a spectrum of disease severity and various degrees of respiratory support. Results included an improvement in mortality from 35% to 31% (rate ratio 0·85; 95% CI 0·76–0·94; p=0·0028) and an impressive drop in median time to being discharged from more than 28 days to 19 days.
In addition, patients who were not receiving invasive mechanical ventilation at randomization were less likely to progress to invasive mechanical ventilation or death. An accompanying editorial that addresses the still unacceptably high mortality figures and the urgent need for additional therapies can be found here.
Newsletter Issue 82, May 3, 2021:
- Preliminary Findings of mRNA Covid-19 Vaccine Safety in Pregnant Persons. 4/21/21. Shimabukuro TT. N Engl J Med.
Early results of mRNA COVID-19 vaccination of pregnant women between 12/14/2020 and 2/28/2021 were obtained from v-safe after-vaccination health checker, v-safe pregnancy registry (patients enrolled by identification through v-safe participation), and VAERS, the vaccine adverse event reporting system. Comparison was to nonpregnant v-safe participants and historical pregnancy outcome statistics. The 35,691 pregnant v-safe participants (94% healthcare workers) reported reactions to vaccination similar to those who were not pregnant. In the registry, 827 pregnancies were completed, 86% with a live birth, and 9% with preterm births. There were 104 spontaneous abortions among the 92 preconception and 1132 first trimester participants. These frequencies are comparable to historical rates. The most common VAERS pregnancy report was spontaneous abortion, reported in 46 patients out of at least 35,691 (0.16%), a rate far lower than published, probably because of underreporting. Although more longitudinal follow-up is necessary, no problems regarding the administration of mRNA COVID-19 vaccine during pregnancy were revealed. - Hospital-Level Variation in Death for Critically Ill Patients with COVID-19. 4/23/21. Churpek MM. Am J Respir Crit Care Med.
This multicenter cohort study utilized the STOP-COVID database to explore the wide variation in published mortality rates for critically ill COVID-19 patients. Data were evaluated on 4019 adult ICU patients admitted to 70 US hospitals between March-June 2020. Thirty-eight percent of patients died within 28 days, with an unadjusted interhospital mortality range of 12-91% (OR 2.06). After mixed-effect regression adjustment for patient- and hospital-level domains, the interhospital range attenuated to 32-44% (OR 1.22). In individual patients, acute physiology contributed 49%, demographics, comorbidities and socioeconomic status 32%, hospital strain and quality 17%, and treatments 3% to mortality risk. The authors emphasized that lower socioeconomic status of the community served by the hospital (characterized by a high percentage of patients who traveled more than 45 min to get to work) is an important contributor to interhospital variability, suggesting that COVID-19 exacerbates disparities in US healthcare. Individual mortality is also impacted by hospital ICU-bed capacity and strain, but treatments had the least impact on outcome variability. [Readers should note that the study reflects an early stage of the pandemic, prior to the positive evidence of steroid therapy on outcome in ventilated patients.]
Newsletter Issue 81, April 28, 2021:
SPECIAL EDITION: Open Critical Care COVID-19 Resources Hub
This is a comprehensive, well-organized, web-based compendium of COVID-19 care and epidemic response guidelines along with learning modules. It summarizes and provides links to COVID-19-care recommendations from the WHO, CDC, NIH, IDSA, SCCM, NEJM, UpToDate, and Partners In Health, among others. The site is led by the UCSF Center for Health Equity in Surgery & Anesthesia with support of the United States Agency for International Development (USAID) and STAR Program, and aims to provide healthcare workers with coordinated, high-quality information and learning tools regarding the care of critically ill COVID-19 patients that are open-access and continuously updated. Each section is also available in Spanish, and some material is available in multiple additional languages.
Sections include the following, along with many more resources:
- COVIDprotocols.org: Adaptable (though not fully peer-reviewed) protocols for the management of COVID-19 patients was created through a collaboration of Brigham & Women’s Hospital, UCSF’s Institute for Global Health Services, Partners in Health, and Open Critical Care. Content is relevant to all practice settings. Topics include testing, infection prevention and control, PPE, patient assessment, outpatient, inpatient and critical care management, obstetrics, pediatrics, and post-COVID care, among others. Features include frequent updates and an “Ask an Expert” chat service. The Spanish language version is here.
- COVID-19 Guidelines Dashboard: This reference tool provides practitioners a quick, intuitively navigable look at current care and pharmacotherapy guidelines from leading healthcare authorities. Stop-light color coding indicates the level of concordance among authorities for each therapy. Date lines and hyperlinked references are included. It promises to be expandable and updated frequently. The Spanish language version is available here.
- Respiratory Care Pocket Card: This link downloads the latest version of a printable, concise summary of oxygen and ventilator therapies, a collaboration of multiple institutions including the UCSF Anesthesia Division of Global Health Equity and USAID. The easy-to-use table format reviews commonly required respiratory care management including non-invasive forms of oxygen delivery, how to calculate ideal body weight, key ventilator modes and settings, how to assess and treat ventilator/patient dyssynchrony, and much more. Relevant references and links are embedded, many (including the card itself) via QR codes that may be useful after printing. The Spanish language version is available here.
- Oxygen Supply and Demand Calculator: This valuable tool for clinicians and administrators balances individual patient use with facility requirements to help maintain a safe O2 supply. It provides an estimate of total facility hourly oxygen consumption and calculates reserves after the user provides the number of hypoxic patients, modes of treatment, and a few details about the oxygen supply infrastructure. Imputed individual patient PO2, PF ratio, and the nonlinear SpO2:FiO2 ratio can be estimated by selecting a patient’s FiO2 and O2 saturation. The Spanish language version is available here.
Newsletter Issue 80, April 26, 2021:
- Genomic characteristics and clinical effect of the emergent SARS-CoV-2 B.1.1.7 lineage in London, UK: a whole-genome sequencing and hospital-based cohort study. 4/12/21. Frampton D. Lancet Infect Dis.
Between 11/9/20 and 12/20/20 samples from 341 COVID-19 inpatients from 2 London hospitals were analyzed with full genomic sequencing. Fifty-eight percent had B.1.1.7 (the UK variant), known to be more transmissible. However in this study the variant was not shown to confer increased risk of severe disease or death compared with non-B.1.1.7 lineages, using the WHO clinical progression ordinal scale. This contrasts with results from other preliminary studies that focused on community cohorts rather than inpatients that appeared to show higher morbidity and mortality among those with B.1.1.7. The study antedates the onset of the UK vaccination program. - The immune response to SARS-CoV-2 and COVID-19 immunopathology – Current perspectives. 4/19/21. Boechat JL. Pulmonology.
These authors review publications through January 2021 on the immunobiology of SARS-CoV-2, virus-receptor interactions, and host immune responses. They focus on impaired innate and acquired immunity as it relates to disease progression and mortality. Immunotherapeutic and pharmacologic strategies targeting both the virus and dysfunctional immune responses are also addressed.
Newsletter Issue 79, April 21, 2021:
SPECIAL EDITION: Vaccine-Induced Immune Thrombotic Thrombocytopenia (VITT)
Robert N. Sladen, MBChB, FCCM on behalf of the IARS Scientific Advisory Board
In recent weeks a great deal of attention has been directed to cases, some of them fatal, of major venous thrombosis associated with thrombocytopenia occurring within 1-3 weeks after initial immunization with the AstraZeneca modified adenovirus vaccine for COVID-19. Although extremely rare relative to the large numbers vaccinated, cases have been more prominent in younger (less than 50 years old), previously healthy patients in Germany and the United Kingdom. In the United States, six similar cases have been reported after immunization with the Johnson & Johnson modified adenovirus vaccine, which has been put on pause pending investigation by the FDA.
In an observational study published in the New England Journal of Medicine (NEJM) on April 16, Dr. Marie Scully and her colleagues report finding pathologic antibodies to platelet factor 4 (PF4) in 22 of 23 patients who suffered thromboembolism and thrombocytopenia after receiving the AstraZeneca vaccine1. Patients also had highly elevated levels of D-dimer with low or normal fibrinogen. The unexpected finding of anti-PF4 antibodies is identical to that of heparin-induced thrombocytopenia (HIT), but in the absence of administered heparin, and is referred to as vaccine-induced immune thrombotic thrombocytopenia (VITT). The mechanism of induction of anti-PF4 antibodies is as yet unknown. As with HIT, use of heparin to treat the thromboses or platelet transfusion for thrombocytopenia worsens thrombosis. Instead, the authors recommend avoidance of platelet transfusions together with a combination of nonheparin anticoagulation and intravenous immunoglobulin and consideration for high-dose glucocorticoids. If there is evidence of thrombosis and thrombocytopenia with elevated D-dimer (>4000 FEU), low or normal fibrinogen and no evidence of an alternate diagnosis, they advocate early treatment as above pending results of anti-PF4 antibody testing by HIT ELISA testing or functional HIT assay.
In an accompanying editorial2, Drs. Cines and Bussel review this and two other reports previously published in the NEJM3,4, a total of 39 patients with cerebral venous sinus, portal, splanchnic or hepatic vein thrombosis who had a 40% mortality. At the current time, a total of 223 possible cases of cerebral venous sinus or splanchnic vein thrombosis have been reported among 34 million recipients of the AstraZeneca vaccine, but not all of these cases have been subjected to rigorous review or tested for anti-PF4 antibodies. The authors point out that we do not yet know how the vaccine elicits the production of anti-PF4 antibodies, nor whether these antibodies are directly responsible for platelet activation and thrombus formation. Anti-PF4 antibodies are detected in 25-50% of patients after cardiovascular surgery, but the incidence of HIT is very uncommon and even then is rarely associated with cerebral venous sinus or abdominal venous thrombosis. The low prevalence of this serious complication must be weighed against the benefits of preventing COVID-19 in the larger population.
On April 16, the American Society of Hematology (ASH) published new guidelines, which will be regularly updated, on the diagnosis and recommended therapy of VITT5. Their recommendation is that urgent evaluation for VITT should be commenced on patients with severe, recurrent or persistent symptoms of headache, abdominal pain, nausea and vomiting, vision changes, shortness of breath, and/or leg pain and swelling that have an onset 4-20 days after vaccination. While VITT has not been reported following the mRNA Pfizer or Moderna vaccines, the ASH recommends immediate evaluation for VITT in any patient presenting with this constellation of symptoms following any COVID-19 vaccination. If initial evaluation reveals thrombocytopenia or thrombosis, an urgent hematology consultation is recommended with avoidance of heparin until VITT has been ruled out.
- Scully M, Singh D, Lown R, Poles A, Solomon T, Levi M, et al. Pathologic Antibodies to Platelet Factor 4 after ChAdOx1 nCoV-19 Vaccination. N Engl J Med. April 2021. https://www.nejm.org/doi/10.1056/NEJMoa2105385.
- Cines DB, Bussel JB. SARS-CoV-2 Vaccine-Induced Immune Thrombotic Thrombocytopenia. N Engl J Med. April 2021. https://www.nejm.org/doi/10.1056/NEJMe2106315.
- Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N Engl J Med. April 2021. https://www.nejm.org/doi/full/10.1056/NEJMoa2104840.
- Schultz NH, Sorvoll IH, Michelsen AE, Munthe LA, Lund-Johansen F, Ahlen MT, et al. Thrombosis and Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. N Engl J Med. April 2021. https://www.nejm.org/doi/full/10.1056/NEJMoa2104882.
- Bussell J, Connors JM, DCines DB, Dunbar CE, Michaelis LC, Kreuziger LB, Lee AYY, Pabinger I. Vaccine-induced Immune Thrombotic Thrombocytopenia: Frequently Asked Questions. American Society of Hematology. Updated April 19, 2021. Accessed April 21, 2021. https://www.hematology.org/covid-19/vaccine-induced-immune-thrombotic-thrombocytopenia.
Newsletter Issue 78, April 19, 2021:
- Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial. 4/12/21. Ramakrishnan S. Lancet Respir Med.
This is a prospective, randomized, open-label, phase-2, parallel-group, age-stratified, 146-patient UK study from July 16 to December 2, 2020 testing inhaled budesonide within 7 days of early symptom development versus standard care. Participants were self-monitored (temperature, pulse oximetry), contacted daily to record symptoms (O2 saturation and temperature), and intermittently self-collected nasopharyngeal swab specimens for analysis. Primary endpoints compared urgent care and emergency room visits and hospitalizations for worsening symptoms, which occurred in 1% of budesonide treated participants, and 14% of the usual care treated group. The study was terminated early with positive results for budesonide inhalant use. Authors concluded that budesonide was effective in treating early COVID-19 infection, could be applicable to global healthcare systems, and that further validation was required. - Editorial: Early treatment with inhaled budesonide to prevent clinical deterioration in patients with COVID-19. 4/12/21. Agusti A. Lancet Respir Med.
This editorial, accompanying the article above, gives perspective to the study and discusses the implications of terminating the study early. The rationale for and use of budesonide (and potentially other inhaled corticosteroids) encourages further trials to confirm the value of this readily available therapy, with significant implications for a cost-effective and easily accessible disease mitigation strategy that could be used globally. - Use of low-molecular weight heparin, transfusion and mortality in COVID-19 patients not requiring ventilation. 4/12/21. Grandone E. J Thromb Thrombolysis.
Prior data has been conflicting with the utility of prophylactic low-molecular weight heparin (LMWH) with COVID-19. This group from Padua retrospectively examined the mortality of 264 non-ventilated inpatients with COVID-19 with respect to the prophylactic use of LMWH enoxaparin. One hundred fifty-six patients (87.7%) received standard LMWH prophylaxis during hospitalization. LMWH was significantly and independently associated with a reduction in mortality in these patients, (OR 0.31, 95% CI 0.13–0.85), as compared to patients who did not receive anticoagulation. Although transfusion or bleeding complications were not higher in these patients, the number of transfusions were significantly and independently associated with mortality. The median fatalities age was 80.5 years. These data suggest that COVID-19 patients who do not require ventilation benefit from prophylactic doses of LMWH.
Newsletter Issue 77, April 14, 2021:
- 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. 4/6/21. Taquet M. Lancet Psychiatry.
This study provides a rich source of data covering a multitude of neurological and psychiatric symptoms in the wake of COVID-19. Using anonymized records from 62, mostly US healthcare organizations, from 3 patient cohorts, one with COVID-19 and two with other contemporaneous illnesses, the authors identify a COVID-specific incidence of neurologic and psychiatric diagnoses of 34% overall, with 13% receiving their first such diagnosis. They convincingly show a link to severity of illness with an incidence of 46% among all patients admitted to ICUs and to a diagnosis of delirium and encephalopathy where the overall incidence rose to 62%. The long-term impact of prolonged recovery due to neurological or psychiatric sequelae of COVID-19 represents a global public health challenge. - Secondary Bacterial Pneumonias and Bloodstream Infections in Patients Hospitalized with COVID-19. 4/6/21. Adelman MW. Ann Am Thorac Soc.
These authors examined the secondary bacterial pneumonias and bloodstream infections (BSI) in 774 patients hospitalized with COVID-19 from February to May 2020. The most common bacteria grown was Staphylococcus aureus. Mortality did not differ between intubated patients with an identified bacterial respiratory pathogen and those without. Overall, mortality was 50% in patients with BSI versus 13.8% without (p<0.0001). These results suggest that hospitalization and central lines are more important than are COVID-19-specific effects in conferring susceptibility to specific pathogens. BSIs in their cohort were also largely related to risk factors, especially central lines, and pathogens associated with hospitalization and did not appear significantly different from the non-COVID data. - Trends in Geographic and Temporal Distribution of US Children With Multisystem Inflammatory Syndrome During the COVID-19 Pandemic. 4/6/21. Belay ED. JAMA Pediatr.
A Center for Disease Control study of patients younger than 21 years old presenting with multisystem inflammatory syndrome in children demonstrating that, although rare (1733 patients countrywide), the presenting symptoms were constant including a rash and conjunctival hyperemia. Half of the affected patients progressed to hypotension and intensive care. These patients tended to be the older of the age range. Most patients were either Hispanic or Black with a median age of 9. The good news was a mortality of only 1.4%.
SPECIAL EDITION: Long COVID
Robert N. Sladen, MBChB, FCCM on behalf of the IARS Scientific Advisory Board
One of the most challenging yet enigmatic aspects of the COVID-19 pandemic is the increasing recognition of prolonged impairment of functional capacity for weeks or months after the initial illness. Like many a “new” entity, it is categorized by a plethora of descriptors, including Long COVID, Long-haul COVID, post-acute COVID-19 Syndrome (PACS) or post-acute sequelae of SARS-CoV-2 infection (PASC). Others simply describe “persistent poor health” or “long-term symptoms.”
Early in the pandemic information on “long-haulers” was spread via social media in the lay community who often met with skepticism from their primary care providers1. Self-reporting by respected physicians has changed attitudes,2, 3 together with published case reports and multiple retrospective studies. Reports from Italy and China revealed symptoms in nearly 90% of discharged hospitalized patients at 60 days4 and 75% at six months,5 but it is now recognized that these symptoms may occur in up to 30% of nonhospitalized patients.6-8 No association has been found between persistent respiratory symptoms in COVID-19 survivors and initial disease severity.9
Reported symptoms run the entire gamut of pathophysiology, the most prominent being chronic fatigue (similar to myalgic encephalomyelitis ME/CFS10), “brain fog,” insomnia, depression, altered mood, dyspnea on exertion, cardiac arrhythmias, anosmia, tinnitus and alopecia among others.11 Physician-sufferers have drawn attention to a myriad chronic or relapsing conditions, including myocarditis, thromboembolic disease, new onset diabetes, thyroiditis and allergies, and autonomic dysfunction such as postural orthostatic tachycardia syndrome (POTS).12
In the US, there is increasing recognition of the impact of Long COVID on healthcare and many clinics have opened to provide support for patients. In May 2020, Mount Sinai Medical Center opened its Center for Post-COVID Care,13 and others have followed. In the United Kingdom, the National Health Service has posted a list of common symptoms14 and provides considerable supportive information on “Your COVID Recovery” on its website. Recently, the Centers for Disease Control (CDC) held a webinar on Clinician Experience with Post-Acute COVID-19 Care.15
Many questions regarding the etiology of Long COVID remain. One of the most perplexing aspects is that many patients with Long COVID have had very mild or even asymptomatic infection.6, 7 Why do some patients develop it and others do not? A large study based on a mobile app suggests that early multisystem symptoms, older age and female sex are predictors of Long COVID.16 Could some long-term symptoms simply represent the lingering physical effects of severe illness and multiorgan injury17 and their psychological consequences such as postintensive care syndrome or PTSD?18, 19 How might these differ from long-term effects after SARS or MERS20 or other historic influenza epidemics?21 Could they be due to persistence of a host viral reservoir even if SARS-CoV-2 testing is negative, or viral fragments that stimulate a persistent aberrant auto-immune response?
A host of published studies have examined aspects of Long COVID, including respiratory, neurologic, auditory and other manifestations. Some even tie in previously unclear syndromes such as POTS.22 Most recently, an extraordinarily comprehensive multidisciplinary review was published in Nature Medicine.11 It provides extensive discussion on available evidence and knowledge for every organ system, a list of relevant studies and publications and also a series of simple, clear graphics that provide an easily understandable overview of the problem. On a practical level, there is increasing awareness that COVID-19 survivors may need ongoing medical and community support, especially in the light of stigma, discrimination, depression and PTSD, akin to cancer survivors.23, 24 Support groups and digital communities for sufferers of Long COVID are forming on social media.25 The UK National Institute for Health and Care Excellence (NICE) has established guidelines for managing the long-term effects of COVID-19.26 The World Health Organization (WHO) has called upon countries to offer more rehabilitation and has developed a clinical platform case report form for “post COVID-19 condition.”27
A number of large-scale research endeavors are being developed to explore Long COVID. The NIH recently announced a $1.15 billion initiative to study PASC.28 In the UK, the Post-Hospitalization COVID Study (PHOSP-COVID) is being led by a national consortium to gather long-term prospective data on 10,000 patients after hospital discharge.29 A 42-country multinational open access study linked to the International Severe Acute Respiratory and emerging Infection Consortium (ISARIC) is being developed to specifically focus on the long-term consequences of COVID-19.30
In sum, there is still much to be learned about the long-lasting effects of COVID-19. What has become abundantly clear is that the personal and public health impact of Long COVID will linger long after the acute pandemic has subsided.
References
- Editorial. Long COVID: let patients help define long-lasting COVID symptoms. Nature. 2020;586:170. https://www.nature.com/articles/d41586-020-02796-2.
- Garner P. Paul Garner: For 7 weeks I have been through a roller coaster of ill health, extreme emotions, and utter exhaustion. thebmjopinion. 2020. https://blogs.bmj.com/bmj/2020/05/05/paul-garner-people-who-have-a-more-protracted-illness-need-help-to-understand-and-cope-with-the-constantly-shifting-bizarre-symptoms/.
- Garner P. Paul Garner: on his recovery from long covid. thebmjopinion. 2021. https://blogs.bmj.com/bmj/2021/01/25/paul-garner-on-his-recovery-from-long-covid/.
- Carfi A, Bernabei R, Landi F. Gemelli Against COVID-19 Post Acute Care Study Group. Persistent Symptoms in Patients After Acute COVID-19. JAMA. 2020;324:603. https://jamanetwork.com/journals/jama/fullarticle/2768351.
- Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220-32. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)32656-8/fulltext.
- Graham EL, Clark JR, Orban ZS, et al. Persistent neurologic symptoms and cognitive dysfunction in non-hospitalized Covid-19 “long haulers”. Ann Clin Transl Neurol. 2021. https://onlinelibrary.wiley.com/doi/full/10.1002/acn3.51350.
- Jacobson KB, Rao M, Bonilla H, et al. Patients with uncomplicated COVID-19 have long-term persistent symptoms and functional impairment similar to patients with severe COVID-19: a cautionary tale during a global pandemic. Clin Infect Dis. 2021. https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciab103/6129932/.
- Logue JK, Franko NM, McCulloch DJ, et al. Sequelae in Adults at 6 Months After COVID-19 Infection. JAMA Netw Open. 2021;4:e210830. https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2776560.
- Townsend L, Dowds J, O’Brien K, et al. Persistent poor health Post-COVID-19 is not associated with respiratory complications or initial disease severity. Ann Am Thorac Soc. 2021. https://www.atsjournals.org/doi/10.1513/AnnalsATS.202009-1175OC.
- Nath A. Long-Haul COVID. Neurology. 2020;95:559-60. https://n.neurology.org/content/95/13/559.
- Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021. https://www.nature.com/articles/s41591-021-01283-z.
- Gorna R, MacDermott N, Rayner C, et al. Long COVID guidelines need to reflect lived experience. Lancet. 2021;397:455-7. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)32705-7/fulltext.
- Center for Post-COVID Care at Mount Sinai. COVID-19 Facts and Resources. 2020. Accessed April 12, 2021. https://www.mountsinai.org/about/covid19/center-post-covid-care.
- NHS. Long-term effects of coronavirus (long COVID). 2021. Accessed April 12, 2021. https://www.nhs.uk/conditions/coronavirus-covid-19/long-term-effects-of-coronavirus-long-covid/.
- Kahn I, COCA, CDC. COCA Call: Treating Long COVID: Clinician Experience with Post-Acute COVID-19 Care. 2021. Accessed April 12, 2021. https://emergency.cdc.gov/coca/ppt/2021/012821_transcript.pdf.
- Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med. 2021. https://www.nature.com/articles/s41591-021-01292-y.
- Marshall M. The lasting misery of coronavirus long-haulers. Nature. 2020;585:339-41. https://www.nature.com/articles/d41586-020-02598-6.
- Sykes DL, Holdsworth L, Jawad N, Gunasekera P, Morice AH, Crooks MG. Post-COVID-19 Symptom Burden: What is Long-COVID and How Should We Manage It? Lung. 2021. https://link.springer.com/article/10.1007/s00408-021-00423-z.
- Naidu SB, Shah AJ, Saigal A, et al. The high mental health burden of “Long COVID” and its association with on-going physical and respiratory symptoms in all adults discharged from hospital. Eur Respir J. 2021. https://erj.ersjournals.com/content/early/2021/02/11/13993003.04364-2020.
- Vittori A, Lerman J, Cascella M, et al. COVID-19 Pandemic Acute Respiratory Distress Syndrome Survivors: Pain After the Storm? Anesth Analg. 2020;131:117-9. https://journals.lww.com/anesthesia-analgesia/pages/articleviewer.aspx?year=2020&issue=07000&article=00019&type=Fulltext.
- Stefano GB. Historical insight into infections and disorders associated with neurological and psychiatric sequelae similar to Long COVID. Med Sci Monit. 2021;27:e931447. https://www.medscimonit.com/abstract/full/idArt/931447.
- Johansson M, Stahlberg M, Runold M, et al. Long-Haul Post-COVID-19 Symptoms Presenting as a Variant of Postural Orthostatic Tachycardia Syndrome: The Swedish Experience. JACC Case Rep. 2021. https://www.sciencedirect.com/science/article/pii/S2666084921001005?via%3Dihub.
- Ernst M, Brahler E, Beutel ME. How can we support COVID-19 survivors? Five lessons from long-term cancer survival. Public Health. 2021. https://www.sciencedirect.com/science/article/pii/S0033350621000123?via%3Dihub.
- Iqbal FM, Lam K, Sounderajah V, Elkin S, Ashrafian H, Darzi A. Understanding the survivorship burden of long COVID. EClinicalMedicine. 2021;33:100767. https://www.thelancet.com/journals/eclinm/article/PIIS2589-5370(21)00047-X/fulltext.
- Yan W. Understanding the Long-Term Impacts of COVID-19 in Survivors. IEEE Pulse. 2021;12:19-23. https://ieeexplore.ieee.org/document/9359551.
- Sivan M, Taylor S. NICE guideline on long covid. BMJ. 2020;371:m4938. https://www.thelancet.com/journals/lanres/article/PIIS2213-2600(21)00031-X/fulltext.
- Wise J. Long covid: WHO calls on countries to offer patients more rehabilitation. BMJ. 2021;372:n405. https://www.bmj.com/content/372/bmj.n405.
- Subbaraman N. US health agency will invest $1 billion to investigate ‘long COVID’. Nature. 2021;591:356. https://www.nature.com/articles/d41586-021-00586-y.
- The Post-hospitalisation COVID-19 study (PHOSP-COVID). Accessed April 12, 2021. https://www.phosp.org.
- Sigfrid L, Cevik M, Jesudason E, et al. What is the recovery rate and risk of long-term consequences following a diagnosis of COVID-19? A harmonised, global longitudinal observational study protocol. BMJ Open. 2021;11:e043887. https://bmjopen.bmj.com/content/11/3/e043887.
Newsletter Issue 75, April 7, 2021:
- Mortality and Readmission Rates Among Patients With COVID-19 After Discharge From Acute Care Setting With Supplemental Oxygen. 4/1/21. Banerjee J. JAMA Netw Open.
A retrospective study of 621 adult COVID-19 pneumonia patients (65% male) who were discharged from inpatient care (76%) or ED (24%) from 2 large urban public hospitals with a carefully executed, patient-focused discharge and follow-up plan showed excellent outcomes. 76% were insured by Medicaid and 84% were Spanish-speaking. Interventions included pre-discharge patient education, non-automated daily telephone contact 7 days/week until not needed, facility-dispensed equipment (pulse oximeter, O2 tank, concentrator), and vendor support. All-cause mortality was low – 1.3% (95% CI, 0.6%-2.5%) with none outside hospital, and 30-day hospital readmission rate was 8.5% (95% CI, 6.2%-10.7%). Median follow-up time was 26 days. Readmission rates were lower than the overall post-acute care 30-day readmission rate (15.2%) for California Dept. of Health Care Services patients in 2020, and compare favorably to privately insured pre-COVID patients in an earlier, referenced report. - New Decade, Old Debate: Blocking the Cytokine Pathways in Infection-Induced Cytokine Cascade. 3/31/21. Rizvi MS. Crit Care Explor.
Providing deep perspective, this narrative review summarizes literature beginning in 1994 evaluating the efficacy and safety of anticytokine therapy for dysregulated immune responses to infectious pathogens. The longstanding idea of neutralizing “cytokine storm” induced by bacterial sepsis and/or ARDS, using cytokine pathway inhibitors or nonpharmacologic cytokine removal has a “grim history.” Severe COVID-19 causes less cytokine release than either condition; however, anticytokine therapy is being used. Discussions include potential reasons for failure, such as the complexity and variation of cytokine cascades, and future directions. - The role of antirheumatics in patients with COVID-19. 4/5/21. Nissen CB. Lancet Rheumatol.
This review, written by an international panel of rheumatologists, nicely summarizes current knowledge of COVID-19 therapy targeting the immune system. Topics include evidence for potentially useful immune modulators (steroids and baricitinib), those under active investigation (tocilizumab, colchicine and anakinra), undergoing early trials (TNF blockade, anti-complement therapy and intravenous immunoglobin) and disproven treatments (hydroxychloroquine). Authors stress that the timing, dosing and interaction of these therapies is incompletely understood, and the hope that studies now underway will provide more clarity. - Toxicity of herbal medications suggested as treatment for COVID-19: A narrative review. 4/5/21. DiPietro MA. J Am Coll Emerg Physicians Open.
The lack of a proven COVID-19 remedy has led to a host of recommendations promoting the use of various plant-based therapeutics, particularly traditional Chinese medicines. Authored by two emergency medicine physicians, this well-researched review of the major characteristics and toxicities of herbal preparations currently in use and sometimes recommended as treatments for COVID-19 provides valuable information on the symptomatology of “toxidromes” caused by mismanagement or overdoses of potentially toxic extracts including oleander and Datura species. - Association between pre-existing respiratory disease and its treatment, and severe COVID-19: a population cohort study. 4/4/21. Aveyard P. Lancet Respir Med.
This retrospective review of medical records from late January through April 2020 of 8,256,161 people registered in 1205 primary care practices in the English NHS showed that people with some respiratory diseases were at an increased risk of hospitalization or death due to COVID-19 compared with those without these diseases with hazard ratios for hospitalization or death respectively as follows: asthma 1·18, 0.99; severe asthma 1·29, 1.08; COPD 1·54, 1.54; bronchiectasis 1·34, 1.12; sarcoidosis 1·36, 1.41; idiopathic pulmonary fibrosis 1·59, 1.47; and lung cancer 2·24, 1.77. The study also provides evidence that the use of inhaled corticosteroids is not associated with a substantially increased risk of severe COVID-19, but nor does it appear to be associated with reduced risk. - COVID-19 Treatment Guidelines
Updated NIH COVID-19 treatment guidelines can be found at this website.
Newsletter Issue 74, April 5, 2021:
- Post-covid syndrome in individuals admitted to hospital with covid-19: retrospective cohort study. 4/1/21. Ayoubkhani D. BMJ.
This is a study of post-COVID syndrome in 47,000+ hospitalized COVID-19 patients individually matched to United Kingdom NHS controls. Patients were discharged by 8/31/2020 and followed for a mean of 140 days, with the study ending on 9/30/2020. The readmission rate was 29% (3.5 times that of controls), and mortality was 12% (7.7 times that of controls). New respiratory disease was 27% more frequent than controls. The risk of diabetes increased 1.5 times, and that of a major cardiovascular event by three times. Younger and ethnic minority patients had greater relative risk than those over age 70. There is an accompanying editorial expressing a need for adjustment of the NHS patient follow-up practices. - ABCDEF Bundle and Supportive ICU Practices for Patients With Coronavirus Disease 2019 Infection: An International Point Prevalence Study. 3/31/21. Liu K. Crit Care Explor.
This is an international, 2-day (June 3 and July 1) survey on the compliance of nutrition, sleep hours and ABCDEF Bundles for 262 COVID-19 patients in 212 ICUs. The authors reported that 47.3% of patients were on mechanical ventilation and 4.6% were on ECMO. Each element of the ABCDEF Bundle was implemented at alarmingly low percentages (16% to 52% compliance), while nutritionally recommended protein was provided to only 50% of ICU patients. Because these supportive measures are known to prevent ICU patients from developing the physical, cognitive and mental disabilities of post-intensive care syndrome, authors strongly suggest that efforts be made to adhere to all evidence-based gold standards of the ABCDEF Bundles including protein supplements and avoiding sleep deprivation in ICU patients. - Escape of SARS-CoV-2 501Y.V2 from neutralization by convalescent plasma. 3/29/21. Cele S. Nature.
Using a live virus-neutralizing assay, investigators tested the effectiveness of convalescent plasma collected from donors during the first (original) and second (S. African variant) waves of COVID-19 against both types of virus. First-wave plasma was effective against first-wave virus, however showed a 15-fold decrease in effectiveness against S. African variant virus. Second-wave plasma was effective against the then-predominant variant strain and, although it demonstrated a 2.3 fold decrease in activity against the original strain, it was still effective. This provides preliminary evidence that vaccines based on variant-of-concern sequences could retain effective activity against other SARS-CoV-2 lineages.
Newsletter Issue 73, March 31, 2021:
- Effect of Helmet Noninvasive Ventilation vs High-Flow Nasal Oxygen on Days Free of Respiratory Support in Patients With COVID-19 and Moderate to Severe Hypoxemic Respiratory Failure: The HENIVOT Randomized Clinical Trial. 3/25/21. Grieco DL. JAMA.
This small, but important, 109-patient, 1:1 ratio, multicenter RCT compares the outcomes of providing non-invasive respiratory support to COVID-19 pneumonia patients with helmet noninvasive ventilation (HNIV) versus high-flow nasal oxygen (HFNO), and shows no significant difference in mortality at 28 days or in the primary end-point which was the number of days free of respiratory support within 28 days of application. It did show a significant decrease in the percent of patients requiring intubation in the HNIV group (30%) versus the HFNO group (51%). A very helpful editorial accompanies the article. - The first 12 months of COVID-19: a timeline of immunological insights. 3/15/21. Carvalho T. Nature Reviews.
Starting with a cluster in Wuhan, China in December 2019, this review summarizes in documentary style, month-to-month key clinical developments and laboratory discoveries over the first year of the COVID-19 pandemic. Many immunologists quickly pivoted from their existing research to study SARS-CoV-2. This brought about a remarkable convergence of knowledge focused on one viral infection. Clinical consortia were formed. In an unprecedented fashion, developments were announced in preprint servers and media to speed information dissemination. The authors prominently showcase the extraordinary leaps in understanding SARS-CoV-2 immune responses and highlight knowledge gaps and areas for future investigations.
Newsletter Issue 72, March 29, 2021:
- Post-acute COVID-19 syndrome. 3/23/21. Nalbandian A. Nat Med.
This comprehensive review of current literature divides post-acute COVID-19 into 2 categories: subacute, lasting 4-12 weeks, and chronic, lasting over 12 weeks. Concise discussions cover post-acute epidemiology, prevention and management of thromboemboli, pulmonary, cardiovascular, neurologic, renal, endocrine, and inflammatory complications as well as race/ethnicity factors, organ system involvement and potential interdisciplinary clinic management, findings from studies of post-acute COVID-19 prevalence, and active research. The need to include rehabilitation in multidisciplinary clinics is reinforced. Links to prominent patient advocacy groups are provided. - Predictors of clinical deterioration in patients with suspected COVID-19 managed in a ‘virtual hospital’ setting: a cohort study. 3/24/21. Francis NA. BMJ Open.
The authors actively followed up on 900 UK COVID-19 patients to determine rates of overnight hospitalization or death over a median period of 21 days from outpatient diagnosis (n=455) or hospital discharge (n=445). 76 patients (8.4%) experienced clinical deterioration. 15 previously hospitalized patients and 3 never-hospitalized patients died, and 58 others required COVID-related hospitalization. Of 35 clinical and laboratory features examined, including O2 saturation, the only predictors of clinical deterioration were increased age (OR 1.04 per year of age), severe renal insufficiency (OR 9.1 for eGFR <30), a history of cancer (OR 2.9), or mental health problems (OR 1.76). - Hospital load and increased COVID-19 related mortality in Israel. 3/26/21. Rossman H. Nature.
An analysis of all 22,636 Israeli COVID-19 inpatients from mid-July 2020 – mid-January 2021 determined that in-hospital mortality increased significantly when >62.5% of the national capacity for severely ill patients (800 beds) was occupied. A validated model using Monte-Carlo methods and a set of Cox regressions was used to predict mortality. Two high-occupancy periods had 22% (SE 3.1%) and 27% (SE 3.3%) greater mortality. Authors postulate that excess mortality during periods of high caseload was most likely due to “an insufficiency of health-care resources.” - Racial and Ethnic Disparities in COVID-19 Incidence by Age, Sex, and Period Among Persons Aged <25 Years – 16 U.S. Jurisdictions, January 1-December 31, 2020. 3/18/21. Van Dyke ME. MMWR Morb Mortal Wkly Rep.
This is a data-rich CDC report on nearly 700,000 COVID-19 cases in young people from jurisdictions representing 23% of the US population. (Included cases represent 77% of total cases due to absent ethnicity data in the remainder.) Incidences among multiple minorities ranged from 0.77 to 4.57 relative to non-Hispanic Whites and disparities evolved during 2020. Large disparities January–April generally decreased May–December, primarily due to higher incidence among Whites. Children <10 rarely tested positive, however incidence increased stepwise from ages 10-24. The largest persistent disparities involved Native Hawaiian and Pacific Islanders, Native Americans, and Hispanics. Ethnic minorities often live in multigenerational homes and include essential workers unable to shelter at home. Equitable and timely access to testing, prevention, and vaccination is urged. - Incidence and mortality due to thromboembolic events during the COVID-19 pandemic: Multi-sourced population-based health records cohort study. 3/12/21. Aktaa S. Thromb Res.
This unique dataset examined the thromboembolic events (TE) in the United Kingdom during a 3-year period. As expected, TE increased with the COVID-19 pandemic and the mortality of TE with COVID-19 was increased as compared to pre-COVID-19. However, the rates of TE deaths in the community also increased. These data suggest that some patients may have avoided the hospital evaluation and that the outpatient evaluation and treatment of patients with COVID-19 may need further investigation. - CHA2DS2-VASc score and modified CHA2DS2-VASc score can predict mortality and intensive care unit hospitalization in COVID-19 patients. 3/17/21. Gunduz R. J Thromb Thrombolysis.
This manuscript examined 1000 Turkish patients admitted with COVID-19 and calculated the CHAD2DS2-VASc score for each patient. They found that this simple score (previously used to assess risk of thromboembolization with atrial fibrillation) was significantly correlated with mortality and the need for ICU admission. The sensitivity and specificity of the original score were 81.7% and 83.9%, respectively. For the modified score sensitivity and specificity were 85.4% and 84.1%. The potential strength of these data lie in the simplicity of the score and the ease of obtaining it.
SAB Comment: These retrospective data would need to be investigated in a prospective fashion before being applied.
Newsletter Issue 71, March 24, 2021:
- Effect of Intermediate-Dose vs Standard-Dose Prophylactic Anticoagulation on Thrombotic Events, Extracorporeal Membrane Oxygenation Treatment, or Mortality Among Patients With COVID-19 Admitted to the Intensive Care Unit: The INSPIRATION Randomized Clinical Trial. 3/18/21. INSPIRATION Investigators. JAMA.
This is a multicenter Iranian study designed to evaluate effects of intermediate (enoxaparin, 1mg/kg qd) vs. standard (enoxaparin 40mg/day) prophylactic anticoagulation in COVID-19 patients admitted to the ICU between July 29 to November 19, 2020. On December 19, the final 30-day follow-up was made to primary outcome of composite venous or arterial thrombosis, treatment with ECMO or mortality within 30 days. The study data and safety monitoring board paused enrollment because of “futility for efficacy and potential excess of safety events, additional clarifications awaited.” The results do not support intermediate-dose prophylactic anticoagulation in unselected COVID-19 ICU patients. - Finding the Optimal Thromboprophylaxis Dose in Patients With COVID-19. 3/18/21. Al-Samkari H. JAMA.
This is a useful, succinct, and well-referenced editorial. It notes the “preponderance of high-quality evidence at this time supports use of standard-dose thromboprophylaxis, not dose escalation, in critically ill patients with COVID-19. However, pending publication of final results from ATTACC, REMAP-CAP and ACTIV-4a multiplatform trial, escalated thromboprophylaxis could be appropriate in moderately ill hospitalized patients with COVID-19 while balancing known comorbidities and bleeding risks.” Together this editorial and accompanying manuscript provide useful insight into current therapeutic concerns regarding appropriate prophylactic anticoagulation regimens for COVID-19 patients prior to and following ICU admission. - Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: a population-level observational study. 3/17/21. Hansen CH. Lancet.
To define the degree to which infection with SARS-CoV-2 confers protection towards subsequent reinfection, the authors analyzed Denmark’s extensive PCR-test data from 2020. Before June, 11,727 people tested positive, and 72 (0.65%) of these again tested positive during the last three months of the year. Protection against repeat infection was 80.5%. Individuals older than 65 years had less protection (47%), and protection for males and females was equal. There was no evidence of waning protection over time (3-6 months vs over 7 months of follow-up). The lower natural immunity in people aged 65 and older underlines the need to vaccinate previously infected individuals in the age group.
SAB Comment: Though similar to a study from the Cleveland Clinic, highlighted in issue 69 of this newsletter, this is a much larger study with different details. - SARS-CoV-2 infection, COVID-19 and timing of elective surgery: A multidisciplinary consensus statement on behalf of the Association of Anaesthetists, the Centre for Peri-operative Care, the Federation of Surgical Specialty Associations, the Royal College of Anaesthetists and the Royal College of Surgeons of England. 3/18/21. El-Boghdadly K. Anaesthesia.
This consensus statement uses evidence from a systematic review and expert opinion to highlight key principles in the timing of surgery. Elective surgery should not be scheduled within 7 weeks of a diagnosis of SARS‐CoV‐2 infection unless the risks of deferring surgery outweigh the risk of postoperative morbidity or mortality associated with COVID‐19. Patients with persistent symptoms of COVID‐19 are at increased risk of postoperative morbidity and mortality even after 7 weeks. Vaccination several weeks before surgery will reduce risk to patients and might lessen the risk of nosocomial SARS‐CoV‐2 infection of other patients and staff.
Newsletter Issue 70, March 22, 2021:
- Changes in Stress and Workplace Shortages Reported by U.S. Critical Care Physicians Treating Coronavirus Disease 2019 Patients. 3/17/21. Gray BM. Crit Care Med.
This article discusses questionnaire responses from 1356 (57%) of polled critical care attending physicians who reported stress graded moderate-high by 67.6% in spring 2020 and 50.7% in fall 2020. Staff shortages were reported by 48.3% in spring with nearly no decrease (46.5%) by fall. Medication and equipment shortages largely improved by fall. However, PPE often remained in short supply; N95 respirator supply was short for 42.5% despite altered practices. Physical and emotional exhaustion rates were high. Elevated patient mortality rates, potential risk of SARS-CoV-2 exposure to personal contacts, risk of personal exposure, patient isolation from their families, and ethical challenges were among the most important drivers.
SAB Comment: For interested readers, detailed results are available in the PDF available via a link in the article or here (http://links.lww.com/CCM/G302). We await updated studies following vaccination of most hospital workers and elderly persons that will likely show further evolution of the incidence of stress in ICU physicians and its drivers. - Expert consensus statements for the management of COVID-19-related acute respiratory failure using a Delphi method. 3/17/21. Nasa P. Crit Care.
This article discusses the Delphi structured communication process used with 39 international experts, which yielded strong suggestions for use of systemic corticosteroids for critical COVID-19. The suggestions include awake self-proning to improve oxygenation and high flow nasal oxygen to potentially reduce tracheal intubation; non-invasive ventilation for patients with mixed hypoxemic-hypercapnic respiratory failure; tracheal intubation for poor mentation, hemodynamic instability or severe hypoxemia; closed suction systems; lung protective ventilation; prone ventilation (for 16–24 h per day) to improve oxygenation; neuromuscular blocking agents for patient-ventilator desynchrony; avoiding delay in extubation for the risk of reintubation; and similar timing of tracheostomy as in non-COVID-19 patients. There was no agreement on positive end-expiratory pressure titration or the choice of personal protective equipment.
Newsletter Issue 69, March 17, 2021:
- Reinfection Rates among Patients who Previously Tested Positive for COVID-19: a Retrospective Cohort Study. 3/15/21. Sheehan MM. Clin Infect Dis.
In a retrospective cohort study, PCR testing in the Cleveland Clinic Health System from March 2020 to February 2021 was analyzed to detect repeat SARS-CoV-2 infection. Of 8,845 individuals with initially positive PCR tests, 62 had reinfections, defined as a positive PCR test at least 90 days following the first positive PCR. Half were asymptomatic, few were hospitalized, and none required intensive care. Protection offered against reinfection was 82%. Risk of reinfection declined with time after initial infection. The authors suggest that the protection afforded by infection with SARS-CoV-2 is adequate to delay vaccination of these people, if vaccine is in short supply.
SAB Comment: A negative PCR test after the first infection was not part of their definition of reinfection therefore, the authors acknowledge that persistent shedding of virus could account for some reinfections. - Increased mortality in community-tested cases of SARS-CoV-2 lineage B.1.1.7. 3/8/21. Davies NG. Nature.
The B.1.1.7 “UK” variant is known to be more infectious. This British study shows it is more lethal. B.1.1.7 is identified with PCR, as the S gene is not amplified: S gene target failure = SGTF. Based on 4,945 deaths within 28 days of community testing of 1,146,534 patients with known SGTF status, authors estimate that the associated adjusted hazard of death is significantly increased across age groups. For example, in 55-69 year old subjects, estimated absolute risk of death within 28 days after a positive test in the community for males increased from 0.6% to 0.9% (95% CI 0.8–1.0%); for females it increased from 0.18% to 0.28% (0.25–0.31%).
SAB Comment: In a separate retrospective British study with similar results ~55,000 adults >30 years old with the B.1.1.7 variant were matched with an equivalent number of controls. It was highlighted in our Newsletter Issue 68, and can be found here. - Antibody Resistance of SARS-CoV-2 Variants B.1.351 and B.1.1.7. 3/8/21. Wang P. Nature.
These investigators report that the “UK variant” (B.1.1.7) remains sensitive to both convalescent plasma and serum collected from vaccinated individuals during Moderna phase I trials (both collected Spring 2020), but refractory to neutralization by most monoclonal antibodies (mAbs) to the spike N-terminal domain (NTD), and relatively resistant to a few mAbs to the receptor-binding domain (RBD). The “South African variant” (B.1.351), containing the E484K mutation is more resistant to neutralization by convalescent plasma (9.4x) and serum from vaccinated individuals (10.3-12.4x). The virus is refractory to most NTD mAbs and multiple individual mAbs to the RBD. This study reinforces concerns about emergent variants and the need for vaccines and mAbs that target them. - Severe covid-19 pneumonia: pathogenesis and clinical management. 3/11/21. Attaway AH. BMJ.
The authors reviewed COVID-19 publications from 1/2020 to 2/2021 and collated the conclusions into a succinct review of major topics descriptive of the disease and its treatment. Concise overviews by topic include mechanism of infection, immunology, pulmonary injury, treatment, outcomes, etc. A table summarizes results of 27 studies regarding respiratory support including high flow nasal cannula, non-invasive ventilation, and invasive mechanical ventilation. Long-term morbidity is also discussed.
Newsletter Issue 68, March 15, 2021:
- Transpulmonary pressure measurements and lung mechanics in patients with early ARDS and SARS-CoV-2. 3/7/21. Baedorf Kassis E. J Crit Care.
To further explore respiratory mechanics in COVID-ARDS, this cohort study from Boston analyzed 40 ventilated patients with chest wall and transpulmonary pressures measured using esophageal pressure monitoring. Lung and respiratory system compliance varied widely over the entire cohort. Elevated basal pleural pressures correlated with increased BMI. Respiratory system and lung mechanics were similar to known existing ARDS cohorts. The wide range of respiratory system mechanics illustrates the inherent heterogeneity that is consistent with typical and COVID-19 ARDS. This information reinforces the practice of treating patients individually, rather than trying to treat with general algorithms.
SAB Comment: Esophageal pressure monitoring, not usually part of routine ventilator care, provides an indirect measurement of intrapleural pressure, which allows one to determine the compliance/elastance of the chest wall separately from transpulmonary pressure. - Timing of surgery following SARS-CoV-2 infection: an international prospective cohort study. 3/10/21. COVIDSurg Collaborative. Anaesthesia.
This prospective cohort study included 140,000 patients undergoing surgery in 1647 hospitals in 116 countries during October 2020. Thirty-one hundred twenty-seven patients with SARS-CoV-2 diagnosed, 0-2, 3-4, 5-6, and >7 weeks prior to surgery were compared with those without SARS-CoV-2. Mortality was increased in all SARS-CoV-2 groups except the >7-week group, as were pulmonary complications. Patients with symptoms lasting >7 weeks also had increased mortality. Mortality in all patients from 0-6 weeks was increased from 1.5% up to 4%. Even asymptomatic patients in the 0-6 week group had increased mortality. Deferring elective surgery for seven weeks, and even longer in the presence of ongoing symptoms is recommended. The report explains statistical methods and includes many graphs. - Risk of mortality in patients infected with SARS-CoV-2 variant of concern 202012/1: matched cohort study. 3/10/21. Challen R. BMJ.
The authors gauged the mortality and future healthcare needs resulting from the new COVID-19 infection variant, B.1.1.7 (VOC-202012/1, from southeast UK in late 2020). The absence of the S gene was found to be a proxy for the B.1.1.7 variant. Patients with this variant were matched for age, sex, ethnicity, and region, with patients with the prior common variants to produce 54,906 pairs. The mortality hazard ratio associated with infection with VOC-202012/1 compared with infection with previously circulating variants was 1.64 in patients who tested positive for COVID-19 in the community. In this comparatively low-risk group, this represents an increase in deaths from 2.5 to 4.1 per 1,000 detected cases. - Attributes and predictors of long COVID. 3/11/21. Sudre CH. Nat Med.
This letter addresses cases of so-called “long COVID” that are rising. These authors examine prevalence and early predictive risk factors. Starting when they were pre-symptomatic, individuals prospectively self-reported symptoms between 3/2020-9/2020 using the COVID Symptom Study app. In 558/4,182 (13.3%) incident cases, symptoms lasted ≥4 weeks; 189 (4.5%) for ≥8 weeks and 95 (2.3%) for ≥12 weeks. Symptoms of fatigue, headache, dyspnea and anosmia increased with age, BMI and female sex. Experiencing >5 symptoms in week 1 predicted long COVID (odds ratio = 3.53 (2.76–4.50)). A simple model identifies at-risk individuals with early symptom patterns for trials of prevention or treatment and plan education and rehabilitation. - Association between ABO blood types and coronavirus disease 2019 (COVID-19), genetic associations, and underlying molecular mechanisms: a literature review of 23 studies. 3/9/21. Zhang Y. Ann Hematol.
International reports generally agree that blood type O is a protective factor. Most, but not all, report that the blood type conferring greatest risk for infection is A. One of the largest retrospective cohort studies indicated risk ratios for infection of 0.87, 1.09, 1.06, and 1.15 for O, A, B, and AB individuals, respectively. Although some report no correlation between blood type and COVID-19 severity or mortality, most studies found that types A and AB had higher risk of severe illness or death, while type O was protective against severe outcomes or death. Potential molecular mechanisms are discussed. - Association of State-Issued Mask Mandates and Allowing On-Premises Restaurant Dining with County-Level COVID-19 Case and Death Growth Rates — United States, March 1–December 31, 2020. 3/5/21. Guy GP. MMWR Morb Mortal Wkly Rep.
During March 1-December 31, 2020, state-issued mask mandates applied in 2,313 (73.6%) of the 3,142 U.S. counties. Mandating masks was associated with a decrease in daily COVID-19 case and death growth rates within 20 days of implementation. During the study period, states allowed restaurants to reopen for on-premises dining in 3,076 (97.9%) U.S. counties. This was associated with an increase in daily COVID-19 case growth rates 41–100 days after implementation and an increase in daily death growth rates 61–100 days after implementation. The study did not distinguish between indoor and outdoor on-premises dining.
Newsletter Issue 67, March 10, 2021:
- The Answer to the Silent “Super Spreader”: An Innovative Way to Manage Chest Drains on Coronavirus Patients With Active Air Leaks. 3/8/21. Angeles C. A A Pract.
In this brief and well-illustrated report, authors propose an innovative mechanism to minimize potential aerosolization of SARS-CoV-2 virus during transportation of patients with chest drains and an active air leak. The main venting of the drain is filtered with an adapted section of an endotracheal tube and airway viral filter while the positive pressure release valve is sealed with bone wax. - Impact of the intersection of anaesthesia and gender on burnout and mental health, illustrated by the COVID-19 pandemic. 3/8/21. Lorello GR. Anaesthesia.
What exactly is “physician burnout”? Does it differ from exhaustion or depression? What is its relationship to gender? These are some of the important questions raised in this review by Canadian authors who discuss the impact of being an anesthesia provider and of gender on professional “burnout” and mental health. The COVID-19 pandemic is used as an example to illustrate how women and men experience stressors and burnout differently. Personal, organizational and governmental factors may contribute to stress as well as its potential amelioration. A useful table is included. - Neurological outcome and quality of life three months after COVID-19: a prospective observational cohort study. 3/8/21. Rass V. Eur J Neurol.
This prospective, multicenter, observational study (n=135) includes patients from 3 categories of disease: severe (n=32 in ICU), moderate (n=72 in COVID-19 ward) and mild (n=31 outpatient). The authors conducted a detailed and systemic evaluation for neurological, psychological, and functional outcomes with a battery of tests at 3 months post COVID-19 infection. The article notes an alarming frequency of central and peripheral nervous systems and functional long-term issues. New onset of neurological disease was found in 20 patients while 82 patients showed neurological signs and symptoms on follow-up. There were disturbing numbers of quality of life, cognitive and functional outcome as well. The majority of those numbers came from ICU patients. - Azithromycin for community treatment of suspected COVID-19 in people at increased risk of an adverse clinical course in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial. 3/4/21. PRINCIPLE Trial Collaborative Group. Lancet.
This study reports the results of the British PRINCIPLE trial, an open armed comparison of azithromycin plus usual care versus usual care alone in outpatients over the age of 65 or over 50 with a comorbidity. Investigators enrolled 540 in the azithromycin arm, 875 controls, and ended the study on November 30, 2020. There was no benefit for time to recovery or prevention of hospitalization. - Effect of Ivermectin on Time to Resolution of Symptoms Among Adults With Mild COVID-19: A Randomized Clinical Trial. 3/4/21. López-Medina E. JAMA.
This double-blind, randomized trial from Colombia, South America studied ivermectin as a treatment for COVID-19. Four hundred seventy-six patients with symptomatic disease (within 7 days of symptom onset, hospitalized or home, up to but not including high flow nasal oxygen) were randomized to 5 days of oral ivermectin or placebo, then followed to day 21. There was no significant difference in the primary outcome (time to resolution of symptoms), nor in secondary outcomes (adverse events and multiorgan failure). The authors state, “The findings do not support the use of ivermectin for treatment of mild COVID-19.”
Newsletter Issue 66, March 8, 2021:
- Comparison of Saliva and Nasopharyngeal Swab Nucleic Acid Amplification Testing for Detection of SARS-CoV-2: A Systematic Review and Meta-analysis. 1/15/21. Butler-Laporte G. JAMA Internal Med.
This systematic review and meta-analysis compared PCR testing (here called “nucleic acid amplification testing – NAAT”) on saliva samples versus nasopharyngeal samples using data from 16 studies. In ambulatory patients with minimal or mild symptoms the pooled saliva NAAT sensitivity (83.2%) and specificity (99.2%) were comparable to the nasopharyngeal swab NAAT sensitivity (84.8%) and specificity (98.9%). Secondary analysis restricted to the 10 peer-reviewed articles gave essentially identical results. Given greater ease and lesser cost of saliva sample collection, the authors propose that saliva NAAT should be prioritized for larger-scale deployment with prospective studies conducted by clinical microbiology laboratories and public health authorities. - Baricitinib Therapy in Covid-19 Pneumonia – An Unmet Need Fulfilled. 3/3/21. Goletti D. N Engl J Med.
This clinically valuable editorial interprets the results of three therapeutic studies. By looking at a scoring system for disease severity the optimal timing for peak benefit from treatment with remdesivir, dexamethasone and baricitinib is defined and an algorithm for therapy is offered. In the ACCT-1 trial, remdesivir was most effective in patients not receiving oxygen or receiving low-flow oxygen. In the RECOVERY trial patients receiving invasive ventilation benefitted most from dexamethasone, however those receiving oxygen also benefitted. In the ACCT-2 trial, the combination of remdesivir and baricitinib was most effective for those receiving high-flow oxygen or noninvasive ventilation. An excellent, clinically useful, graphic defines stages of severity and optimal treatment windows along with a typical timeline of days since symptom onset. - Predictors of failure with high-flow nasal oxygen therapy in COVID-19 patients with acute respiratory failure: a multicenter observational study. 3/6/21. Mellado-Artigas R. J Intensive Care.
The authors developed a simple online tool to predict the eventual need for intubation and mechanical ventilation in patients treated with high-flow nasal oxygen (HFNO) based on the outcome of 259 patients from 36 Spanish and Andorran intensive care units. Inputs required include those from the SOFA score (platelet count, bilirubin, mean arterial pressure, Glasgow Comma Scale, creatinine, and FiO2) plus SpO2 and respiratory rate. Performance measured by area under the curve was 0.88, (95% CI 0.80-0.96). - Altered pulmonary blood volume distribution as a biomarker for predicting outcomes in COVID-19 disease. 2/26/21. Morris MF. Eur Respir J.
This group examined the CT scans of 313 COVID-19 patients and used an automated program to obtain the percentage of blood vessels with a cross-sectional area 1.25–5 mm2 (BV5%). If the (BV5%) was < 25 %, the data suggested odds ratio (OR) 5.58 for death, and OR 3.20 for intubation. Decreased BV5% has been noted in prior literature in patients with COPD and ARDS. While the decrease in BV5% may represent a change associated with ARDS, this novel marker is noteworthy and merits further investigation.
SAB Comment: This is a tool we have not seen before, and if validated, may become clinically relevant due to the high-observed odds ratio for mortality and death.
Newsletter Issue 65, March 3, 2021:
- Acute Respiratory Distress Syndrome: Contemporary Management and Novel Approaches during COVID-19. 2/4/21. Williams GW. Anesthesiology.
This is a concise yet comprehensive review of 25 years of ARDS intervention trials, primarily supported through the US ARDS and the Prevention and Early Treatment of Acute Lung Injury (PETAL) Networks and the RECOVERY trial in the UK. Although outcomes and understanding of ARDS has improved significantly, not all interventions studied resulted in clinical benefit and some were potentially harmful. In addition to the discussion, figures and a table nicely summarize findings from pre-COVID-19 and recent reports. - Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19. 2/25/21. The REMAP-CAP Investigators. New Engl J.
Focusing on therapeutic success for COVID-19 patients requiring organ support measures, 895 patients were randomly assigned to receive one of two IL-6 receptor antagonists and were compared to a 402-patient control group receiving standard care, including glucocorticoids and antivirals. Tocilizumab was given to 366 patients starting in April, and sarilumab, available only since June, to 49 patients. Statistical criteria for efficacy were met in October 2020 and demonstrated significant therapeutic benefit for the primary outcome, expressed in more organ-support free days and lower in-hospital mortality. Both drugs also improved secondary outcomes, including 90-day survival, time to ICU and hospital discharge, among others. Authors link the success of IL-6 antagonists in this series to their use in the sickest patients while organ dysfunction is still reversible. - “Silent” Presentation of Hypoxemia and Cardiorespiratory Compensation in COVID-19. 2/4/21. Bickler PE. Anesthesiology.
This well-written review discusses variability in the human response to hypoxemia from any cause, based upon longstanding research in both normal subjects and patients with pathologic conditions. The theory that COVID-19 is unique in its ability to cause hypoxemia without dyspnea (so-called “happy hypoxia”) is refuted, and the physiologic basis for this somewhat surprising condition is explained. When oxygen concentration falls, the most important compensatory mechanism to preserve oxygen delivery is augmentation of cardiac output. If cardiac reserve is compromised, patients experiencing profound hypoxemia are at increased risk for hypoxic organ damage and death. - Development of Severe COVID-19 Adaptive Risk Predictor (SCARP), a Calculator to Predict Severe Disease or Death in Hospitalized Patients With COVID-19. 3/1/21. Wongvibulsin S. Ann Intern Med.
This article presents a simple web-based calculator for the risk of developing severe disease (requiring high-flow nasal oxygen, non-invasive or mechanical ventilation) or of death in the following day or in the following week. The input values include only the worst O2 saturation, and highest O2 flow rate in the last 6 hours and in the last 24 hours, plus, for milder cases, the absolute neutrophil and lymphocyte count. Development was facilitated by a machine learning tool used to analyze 105 parameters from 3294 patients hospitalized from May to December in Baltimore area hospitals. The final calculator requiring only the above few measures showed an area under the curve (AUC) of 0.89 for one-day predictions and 0.83-0.87 for one-week predictions. - Acute covid-19 and multisystem inflammatory syndrome in children. 3/2/21. Rubens JH. BMJ.
This is a clinical summary of children with acute COVID-19 and the associated multiple inflammatory syndrome in children (MIS-C). A small proportion of children go on to develop severe acute COVID-19 disease and require hospitalization because of respiratory compromise or complications of SARS-CoV-2 infection. Clinicians should consider MIS-C in children presenting with fever and abdominal symptoms, particularly if they develop conjunctivitis or rash, and refer to a pediatric emergency department for evaluation. MIS-C can have overlapping symptomatology with disease processes that require prompt treatment, such as sepsis, toxic shock syndrome, myocarditis, and meningitis.
Newsletter Issue 64, March 1, 2021:
- Progesterone in Addition to Standard of Care Versus Standard of Care Alone in the Treatment of Men Hospitalized with Moderate to Severe COVID-19: A Randomized, Controlled Pilot Trial. 2/23/21. Ghandehari S. Chest.
This is a small, early pilot study based on the observation that men are faring worse than women when contracting severe COVID-19. It also discusses the theory that progesterone may modulate the immune response to the SARS-CoV-2 virus, decrease severity of illness and shorten the need for supplemental oxygen. A group of 42 men with respiratory symptoms of COVID-19 were randomized 1:1 to receive a 5-day course of progesterone or standard care. Over a 7-day observation period, the progesterone group improved significantly by 1.5 points on a 7-point clinical status scale. Other results of 2.5 fewer days in the hospital and 3 fewer days on supplemental oxygen did not reach significance. There were no side effects, specifically no increase in thromboembolic events. - Incidence of venous thromboembolism among patients with severe COVID-19 requiring mechanical ventilation compared to other causes of respiratory failure: a prospective cohort study. 2/18/21. Pellegrini JAS. J Thromb Thrombolysis.
This paper examines 57 ICU patients admitted with COVID-19 and compares them to 13 ICU patients without COVID-19. All the patients had lower extremity ultrasonography scans on days 3, 7 and 14. The incidence of deep venous thrombosis, pulmonary embolism, and thrombosis at the central catheter insertion sites was higher in those with COVID-19 as compared to those without (36.8% vs. 0%, p = 0.023). Independent risk factors for venous thromboembolism were COVID-19 and D-dimers. These data help establish the incidence of thrombosis in ICU patients with COVID-19 with a rigorous standard protocol.
SAB Comment: These data are some of the best quality data examining the incidence of venous thrombosis in ICU patients with COVID-19. - Intensive care management of patients with COVID-19: a practical approach. 2/19/21. Hajjar LA. Ann Intensive Care.
This is an excellent, comprehensive and refreshing examination and review of the major organ system and pathophysiology. It illustrates management at each corresponding steps of pathology with a few, very clear infographics to support it. The article lives up to the title: practical management of COVID-19 in the ICU. - The Pathophysiology and Dangers of Silent Hypoxemia in COVID-19 Lung Injury. 2/23/21. Swenson KE. Ann Am Thorac Soc.
COVID-19 patients who develop minimally symptomatic hypoxemia are at risk for rapid decompensation, but the pathophysiology of silent hypoxemia in COVID-19 lung injury remains inadequately explained. This review describes the mechanisms, either viral-induced or within the broad normal range of hypoxic sensitivity in the lung and nervous system in healthy people, which could lead to profound hypoxemia without apparent dyspnea, based on what is currently known about SARS-CoV-2 and normal respiratory physiology and pathophysiology in other forms of ARDS. Three aspects are addressed: differences in lung compliance, pulmonary vascular responses to hypoxia, and nervous system sensing and response to hypoxemia. All could be inextricably linked to the phenomenon of silent hypoxemia. - Intracerebral Hemorrhage in COVID-19 Patients with Pulmonary Failure: A Propensity Score-Matched Registry Study. 2/23/21. Lang CN. Neurocrit Care.
Some reassurance comes from this retrospective study of all patients 18 years old and older admitted to a tertiary care hospital from 2018 to May 2020 comparing the incidence of intracerebral hemorrhage (ICH) between COVID-19 and non-COVID-19 ARDS patients. The authors found that the 19% incidence of ICH in the 47 COVID-19 ARDS patients was not significantly higher than the 11% incidence in the 116 non-COVID-19 ARDS patients despite higher rates of therapeutic anticoagulation and antiplatelet therapy in the COVID-19 patients.
Newsletter Issue 63, February 24, 2021:
- CORRECTION: In Newsletter Issue 62, there was an error in the summary for the article, Six-Month Survival After Extracorporeal Membrane Oxygenation for Severe COVID-19. This is the corrected statement: A pH > 7.23 and age under 60 years resulted in survival rate over 50%, while an 80% overall mortality was noted in severely acidotic, older patients.
- Effect of High-Dose Zinc and Ascorbic Acid Supplementation vs Usual Care on Symptom Length and Reduction Among Ambulatory Patients With SARS-CoV-2 Infection: The COVID A to Z Randomized Clinical Trial. 2/12/21. Thomas S. JAMA Netw Open.
Four groups of out-patients with confirmed COVID-19 were randomized to receive either zinc 50 mg, ascorbic acid 8 grams, a combination of both, or usual care, and were asked to report a 50% reduction of symptoms as a primary endpoint. Between late April and mid-October 2020, when the trial was stopped, a total of 214 patients in the Cleveland Clinic system in Ohio and Florida were enrolled. No difference in primary outcome between the groups was detected.
SAB Comment: The linked editorial adds perspective to the role of supplements and highlights value and shortcomings of this RCT. - D-dimer and Death in Critically Ill Patients With Coronavirus Disease 2019. 2/16/21. Short SAP. Crit Care Med.
This is a study of 68 hospitals across America demonstrating that D-dimers, the result of cross-linked fibrin degradation, correlates to the prognosis of death. In a population of 3418 studied with an average age of 62 years, the majority males and significant numbers with hypertension and diabetes mellitus, the risk of death increased with level of D-dimer elevation as measured during the first 2 days of ICU admission. Patients were followed as close as possible to 90 days with an overall mortality of 39.6%. Most patients who died, 34.5% of all patients, did so in the first 28 days. The authors are careful to state this study is not intended to make recommendations for anticoagulation but only as a risk stratification for mortality. - Oral Complications of ICU Patients with COVID-19: Case-Series and Review of Two Hundred Ten Cases. 2/9/21. Hocková B. J Clin Med.
Oral complications are common in long-term ICU COVID-19 patients requiring prolonged prone positioning and mechanical ventilation. This three patient case series and accompanying literature review of 210 cases suggests not only traumatic complications associated with positioning and mechanical ventilation (report re: intubation versus tracheostomy) but also use of antibiotics, antivirals and steroids plus difficulties associated with performing effective oral toilet as causative factors. Authors recommend a multidisciplinary approach in severe cases including dermatology, oral surgery and dentistry to study causation and routine monitoring and management of at-risk patients. - Outcomes of Patients With Coronavirus Disease 2019 Receiving Organ Support Therapies: The International Viral Infection and Respiratory Illness Universal Study Registry. 2/8/21. Domecq JP. Crit Care Med.
Through the Society of Critical Care Medicine platform, data was collected 2/15/20-11/30/20 on 20,608 hospitalized COVID-19 patients from 168 hospitals in 16 countries. This article reports mortality of hospitalized COVID-19 patients according to age and type(s) of organ support therapy. For centers reporting >10 intubated patients, risk and age adjusted mortality averaged 52.6% but ranged from 24% to 76%. Main drivers of mortality and discharge to home were duration of hospital stay, ICU admission, age, number of comorbidities, and number of organ degree support (MV, MV + Vaso active drugs, MV + RRT, MV+ RRT+ Drugs, ECMO). - Surviving Sepsis Campaign Guidelines on the Management of Adults With Coronavirus Disease 2019 (COVID-19) in the ICU: First Update. 2/8/21. Alhazzani W. Crit Care Med.
This is an update of the published Society of Critical Care Medicine (SCCM) Surviving Sepsis Guidelines for COVID-19 therapy and management. A multinational task force (43 experts; 14 countries) reviewed guidelines; three new and six revised are included. Rationale was included in discussions. This is a good reference and resource for latest SCCM guidelines, incorporating the best available evidence. - mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. 2/3/21. Wang Z. Nature.
This basic science article in Nature showed similar antibody and memory B cell responses in volunteers who received mRNA vaccinations vs those naturally infected. Of note, against emerging variants, whole plasma (containing many diverse antibodies) remained active, neutralizing, and protective, though reduced in both groups. Monoclonal antibodies (mAbs) from laboratory-derived single B cells remain active in neutralizing current virus. However, against emerging mutants, neutralization by the most potent single mAbs was reduced/abolished. Further, when single mAbs were placed in extended virus co-culture, virus mutations resulted. This suggests that clinically, caution is warranted in treating with single mAb infusions rather than mAb combinations as single mAbs may drive virus mutations.
Newsletter Issue 62, February 22, 2021:
- Six-Month Survival After Extracorporeal Membrane Oxygenation for Severe COVID-19. 2/12/21. Biancari F. J Cardiothorac Vasc Anesth.
An international study done early in the pandemic of 132 COVID-19 patients defined the conditions for optimal survival at 6 months after ECMO. Ninety-two percent of the patients received venovenous canulation. A Ph >7.23 and age under 60 years resulted in survival over 50% while an 80% overall mortality was noted in severely acidotic, older patients. Sixty-three percent of the 70 deaths reported occurred during ECMO. - Thrombotic complications in 2928 patients with COVID-19 treated in intensive care: a systematic review. 2/15/21. Jenner WJ. J Thromb Thrombolysis.
This is a systematic review of ICU patients with COVID-19. The article reviewed 28 studies with 2,928 patients. The incidence of thrombotic events was 34%, but studies employing systematic screening reported a significantly higher incidence of venous thrombosis compared to those relying on clinical suspicion alone (56.3% vs. 11.0%, p < 0.001) despite anticoagulant thromboprophylaxis. Consideration should be given to systematic screening and increased dose anticoagulant thromboprophylaxis in patients with COVID-19 in the ICU.
SAB Comment: Two limitations of the review include that the prophylaxis and the screening protocols were not uniform. - Early initiation of prophylactic anticoagulation for prevention of coronavirus disease 2019 mortality in patients admitted to hospital in the United States: cohort study. 2/12/21. Rentsch CT. BMJ.
Of 4297 patients admitted to the Veterans’ Administration system with COVID-19, 3627 (84.4%) received prophylactic anticoagulation within 24 hours of admission. Early initiation of prophylactic anticoagulation compared with no anticoagulation among patients admitted to hospital with COVID-19 was associated with a 27% decreased risk of 30-day mortality. Receipt of prophylactic anticoagulation was not associated with increased risk of bleeding that required transfusion. These findings provide strong real-world evidence to support guidelines recommending the use of prophylactic anticoagulation as initial treatment for patients with COVID-19 on hospital admission. - Should Remdesivir Be Used for the Treatment of Patients With COVID-19? Rapid, Living Practice Points From the American College of Physicians (Version 2). 2/9/21. Qaseem A. Ann Intern Med.
This clinical guideline updates the 10/5/2020 version, based on a U.S. Dept. of Veterans Affairs Evidence Synthesis Program review through 12/7/2020. Target patients include all hospitalized, non-pregnant, adults with COVID-19. Current evidence suggests an overall modest net benefit of remdesivir for patients who require oxygen but not invasive mechanical ventilation (IMV) or ECMO at initiation, and suggests that 5-day treatment may be as effective as 10 days, without increased harm. Recovery is modestly better and more rapid and serious adverse events are modestly reduced. Mortality is slightly reduced. Evidence quality was low-moderate in all RCTs.
Guideline:- Begin 5 days of remdesivir for hospitalized patients requiring oxygen but not IMV or ECMO.
- Extend remdesivir to 10 days if IMV or ECMO is initiated during the 5-day course.
SAB Comment: A NEJM study published 12/11/20, titled, Baricitinib plus Remdesivir for Hospitalized Adults with Covid-19, and highlighted in our newsletter issue 47, indicates that dual therapy with remdesivir and the anti-inflammatory baricitinib was found to be superior to remdesivir alone.
- Static compliance of the respiratory system in COVID-19 related ARDS: an international multicenter study. 2/9/21. Vandenbunder B. Crit Care.
This multicenter, prospective observational study in 21 ICUs in France and Belgium in early Spring compared respiratory mechanics in 372 ventilated COVID-19 patients on Day 1 and Day 14 (when possible) in an effort to predict outcome at 28 days. The mean respiratory system compliance (Crs) on day 1 was 38 +/- 13 ml/cmH2O, with a monomodal distribution, similar to ARDS of other causes. Crs decreased between Day 1 to Day 14 but the decrease was not associated with Day 28 outcome. Higher compliance values on Day 1 were not associated with faster weaning of mechanical ventilation nor with improved survival.
SAB Comment: Despite reporting a median compliance of 38 ml/cm H2O, we treat individuals according to their own specific physiology that may not reflect the median. As recommended in multiple guidelines, respiratory therapy should be tailored at an individual level.
Newsletter Issue 61, February 17, 2021:
- Perspectives of Ketamine Use in COVID-19 Patients. 1/1/21. Weinbroum A. J Korean Med Sci.
This article is a brief review from Israel of the use of ketamine sedation in the context of COVID-19. It includes the rationale, practical aspects and potential benefits such as evidence of reduced IL-6 and CRP following infusion. Advantages include minimal hemodynamic suppression, bronchodilation, and lack of respiratory depression during spontaneous or assisted ventilation. Authors also discuss a potentially lower incidence compared with other sedatives of psychological complications including acute anxiety and post-traumatic stress and depression following illness. - Association of chronic anticoagulant and antiplatelet use on disease severity in SARS-COV-2 infected patients. 2/2/21. Ho G. J Thromb Thrombolysis.
This article reviews data from Kaiser Permanente Northern California, which covers 4.4 million patients, and examined the records of the 28,076 patients with confirmed positive SARS-CoV-2 infection. 1% were prescribed anticoagulants within 3 months prior to diagnosis and 3% were taking antiplatelet agents. Neither was associated with a reduced risk of hospitalization, venous thromboembolism, emergency department visit, ICU stay, invasive ventilation or death. Based upon these data, authors do not recommend broad institution of anticoagulation or antiplatelet therapy for patients testing positive for SARS-CoV-2 infection. - Neutralizing Monoclonal Antibody for Mild to Moderate COVID-19. 1/21/21. Malani PN. JAMA.
This editorial compares the BLAZE-1 trial results published in January 2021 with an interim analysis published online in October 2020. Because final data resulted in changes in the effect sizes for all 5 cohorts, earlier results indicating that one dose cohort made a significant difference had to be overturned. Authors note that in normal times an interim analysis of an ongoing clinical trial would not have been published. The FDA has issued emergency use authorizations for both bamlanivimab (Lilly) and for the combination of casirivimab and imdevimab (Regeneron) for outpatients with mild to moderate symptoms of COVID-19 and risk factors for progression to severe disease (such as advanced age, obesity, diabetes, chronic kidney disease, and immunosuppression). - Cardiopulmonary Resuscitation in the Prone Position in the Operating Room or in the Intensive Care Unit: A Systematic Review. 2/1/21. Anez C. Anesth Analg.
The discussion section in this article provides an excellent, practical “how-to,” including diagrams, for prone CPR and defibrillation. This review is based on 52 selected pre-COVID-19 articles including case reports of 14 intubated patients (13 during surgery). The data presented confirms that CPR in the prone position is a reasonable alternative to supine CPR when the latter cannot be immediately implemented, and the airway is already secured. Defibrillation in the prone position is also possible. - Mortality and renal outcomes of patients with severe COVID-19 treated in a provisional intensive care unit. 1/21/21. Hittesdorf E. J Crit Care.
This study involves 116 COVID-19 patients who required mechanical ventilation and were cared for in an OR-ICU. The patients were followed for 90 days for mortality and renal outcomes. 30.2% died (n=35). Mortality among 45 patients receiving continuous replacement therapy (CRRT) was 40% (n=18) vs. 23.4% (n=17) in 71 patients who did not receive CRRT. The stage of AKI did not affect mortality compared with no AKI. However, those with stage 3 were more likely to require CRRT and to die during hospitalization. Only two survivors required dialysis at 90 days and outcomes did not differ from those cared for in a regular ICU.
Newsletter Issue 60, February 15, 2021:
- Transmission of COVID-19 in 282 clusters in Catalonia, Spain: a cohort study. 2/2/21. Marks M. Lancet Infect Dis.
In an attempt to identify variables that affect the transmission dynamics of SARS-CoV-2, these investigators from Spain analyzed contact tracing data associated with a randomized control trial. Quantitative RT-PCR and clinical data was analyzed on 282 adult, non-hospitalized index cases with a total of 753 contacts. The viral load of the index case was the leading determinant of the risk of PCR positivity among contacts and viral load significantly influenced the risk of developing the symptomatic disease in a dose-dependent manner. No association of risk of transmission was found with reported mask usage by contacts, with the age or sex of the index case, or with the presence of respiratory symptoms in the index case. - Occurrence and Timing of Subsequent Severe Acute Respiratory Syndrome Coronavirus 2 Reverse-transcription Polymerase Chain Reaction Positivity Among Initially Negative Patients. 2/5/21. Long DR. Clin Infect Dis.
The authors compared the occurrence of a discordant result of RT- PCR in two health systems. They assessed the conversion rate to a new positive in less than 7 days. They noted the conversion rate was at 3.5% (4.1% at the University of Washington, 2.8% at Stanford). Retesting was done based on clinical symptoms of patients. These observations suggest that false-negative RT-PCR results do occur, but at a low frequency. Neither team was able to calculate a true clinical sensitivity or false-negative proportion due to the lack of a gold-standard. - Rapid decline of neutralizing antibodies against SARS-CoV-2 among infected healthcare workers. 2/8/21. Marot S. Nature Communications.
Serological testing is used to identify individuals who are immunized and potentially “protected” against re-infection. From 28 January to 21 March 2020, 26 healthcare workers from Pitié-Salpêtrière University Hospital in France were enrolled in this study. Healthcare workers with mild COVID-19 were tested three weeks (D21), two months (M2) and three months (M3) after the onset of symptoms. All healthcare workers displayed seroconversion at D21 after symptom onset, and elicited a neutralizing antibodies response to SARS-CoV-2 correlated with the anti-receptor binding domain antibody levels. However, this neutralizing activity declines, and may even be completely lost, in association with a decrease in systemic IgA antibody levels from 2 months after disease onset. - SAB Comment: The SAB policy is to only review articles that have undergone peer review for inclusion in this newsletter. We are making an exception for the following two studies due to their therapeutic implications for COVID-19 patients. Final versions of these studies will be presented as they become available.
- ATTACC, ACTIV-4a & REMAP-CAP multiplatform RCT: Results of interim analysis. 1/28/21. NHLBI.
This is new, exciting RCT data for decreasing morbidity and mortality for COVID-19 with therapeutic anticoagulation. This interim data from NHLBI examines 3 international trials, suggesting decreased morbidity and mortality for COVID-19 patients with therapeutic anticoagulation for patients not in the ICU. - Inhaled budesonide in the treatment of early COVID-19 illness: a randomised controlled trial. 2/8/21. Ramakrishnan S. medRxiv.
This open label trial from Oxford, UK, convincingly showed that a steroid inhaler used twice daily within 7 days of onset of mild COVID-19 significantly improves outcome measured primarily in hospitalization and secondarily in days to recovery from symptoms, fever and low oxygen saturation. Statistically, the difference in proportions was 0.131, 95% CI (0.043, 0.218), p=0.004, indicating a relative risk reduction of 90% for patients using the budesonide inhaler compared to usual care.
SAB Comment: This study has not yet been peer reviewed, was partially funded by Astra Zeneca and was halted early due to the December surge in COVID-19 cases in the study area. However, the authors plead convincingly that this ubiquitous treatment modality can influence the course of illness and possibly avoid prolonged recovery from SARS-CoV-2.
- ATTACC, ACTIV-4a & REMAP-CAP multiplatform RCT: Results of interim analysis. 1/28/21. NHLBI.
Newsletter Issue 59, February 10, 2021:
- Azithromycin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. 2/5/21. RECOVERY Collaborative Group. Lancet.
As part of the British RECOVERY trial which includes 176 hospitals, 2582 hospitalized patients with COVID-19 were randomized to receive azithromycin 500 mg daily for 10 days and compared to 5181 patients receiving standard care. The 28-day all-cause mortality for both patient groups was 22% indicating that azithromycin has no benefit for COVID-19 and should be used for antimicrobial indications only.
SAB Comment: The Randomised Evaluation of COVID-19 Therapy (RECOVERY) trial is an investigator-initiated, individually randomized, controlled, open-label, adaptive platform trial to evaluate the effects of potential treatments in patients admitted to hospital with COVID-19. After completing work on azithromycin, dexamethasone, hydroxychloroquine, lopinavir–ritonavir, convalescent plasma, and tocilizumab, study into the effects of REGN-COV2 (a combination of two monoclonal antibodies directed against SARS-CoV-2 spike glycoprotein), aspirin, and colchicine are still underway. - Extracorporeal membrane oxygenation in patients with severe respiratory failure from COVID-19. 2/2/21. Shaefi S. Intensive Care Med.
This article reports the results of a study on the use of V-V ECMO in selected COVID-19 patients treated in experienced centers with mortality reduction (66.8% survival at 60 days). The study represents ECMO patients admitted across all participating ICUs during the trial period (190/5122). The article includes detailed analysis and an interesting use of an emulation cohort to support conclusions, eliminate confounders and immortal time bias and provide comparator between ECMO and non-ECMO patients to provide a non-ECMO control group lacking in prior studies. The author emphasizes the importance of early initiation in carefully selected patients treated in experienced centers to maximize outcomes. - Evolution of antibody immunity to SARS-CoV-2. 1/18/21. Gaebler C. Nature.
SARS-CoV-2 neutralizing antibody levels eventually decrease post-illness or vaccination. It is unknown how well memory B cells produce antibodies many months later. Eighty-seven individuals were assessed at 1.3- and 6.2-months post-infection. As expected, IgM, and IgG anti-SARS-CoV-2 spike protein receptor binding domain (RBD) antibody titers decreased significantly. Functionally, plasma viral killing activity decreased fivefold. However, at 6.2 months, memory B-cells remained unchanged and continued evolving antibodies showing antibody sequence changes with increased potency and resistance to RBD mutation. Following up on known stool SARS-CoV-2 persistence, the authors related ongoing memory B-cell evolution to lingering antigen immunoreactivity shown in intestinal biopsies 4 months post-infection. - Global absence and targeting of protective immune states in severe COVID-19. 1/25/21. Combes A. Nature.
This fascinating study shows that immune response to COVID-19 is complex and differs between severe systemic effects in some patients and milder symptoms in others. Authors exposed the differences by studying whole blood analysis identifying individual cellular elements and expression in samples of severe and mild disease. Examination of serum in mild disease shows production of interferon-stimulated genes which blunt overproduction of anti-SARS-CoV-2 antibodies which in severe disease are higher and associated with lower viral titers than seen in mild disease. The authors make research suggestions to study modification of this response. - SARS-CoV-2 infects and replicates in cells of the human endocrine and exocrine pancreas. 2/4/21. Müller JA. Nat Metab.
These investigators show that SARS-CoV-2 can cause diabetes in the absence of autoantibodies and other pancreatic disorders by directly infecting human exocrine (enzyme-producing) and endocrine (hormone-producing) pancreatic cells. Beta-cells (insulin-producing) express ACE-2, and TMPRSS2 allowing entry and then viral replication, inhibitable by remdesivir. The reduction in insulin-secretory granules in beta-cells results in reduced glucose-stimulated insulin secretion. The nucleocapsid protein was detected in four post-mortems in exocrine cells, beta-cells and in close proximity to the islets of Langerhans. These data suggest that SARS-CoV-2 targeting the pancreas leads to endocrine dysregulation (hyperglycemia, DKA, new onset Type-1 diabetes) and pancreatitis (up to 33%).
Newsletter Issue 58, February 8, 2021:
- The effect of early treatment with ivermectin on viral load, symptoms and humoral response in patients with non-severe COVID-19: A pilot, double-blind, placebo-controlled, randomized clinical trial. 1/19/21. Chaccour C. eClin Med.
Ivermectin has been shown to inhibit SARS-CoV-2 replication in vitro. This 24-patient pilot randomized, double-blind, placebo-controlled trial was launched in Spain to determine whether a single 400mcg/kg dose of ivermectin could provide a public health benefit by limiting viral spread when administered early to young patients attending the emergency room with symptoms compatible with COVID-19 and with no more than 72 h of fever or cough. No difference in the proportion of PCR positives were found but a marked reduction of self-reported anosmia/hyposmia, a reduction of cough and a tendency to lower viral loads and lower IgG titers was noted. A significantly faster recovery from anosmia (76 v 158 days) is among the results and – along with multiple retrospective studies showing favorable outcomes – calls for further investigations into the potential benefits of this widely available drug. - SARS-CoV-2 viral load is associated with increased disease severity and mortality. 10/30/20. Fajnzylber J. Nature Communications.
The authors quantified SARS-CoV-2 viral load from participants with a diverse range of COVID-19 disease severity, including those requiring hospitalization, outpatients with mild disease, and individuals with resolved infection. Amongst participants hospitalized with COVID-19, the authors report that a higher prevalence of detectable SARS-CoV-2 plasma viral load is associated with worse respiratory disease severity, lower absolute lymphocyte counts, and increased markers of inflammation. Forty-four percent of those on a ventilator had detectable viremia compared to 19% of those receiving supplemental oxygen by nasal cannula and 0% of individuals not requiring supplemental oxygen. Compared to individuals who were discharged from the hospital, those who eventually died had significantly higher levels of plasma viremia at the time of initial sampling. - The Association of Preinfection Daily Oral Anticoagulation Use and All-Cause in Hospital Mortality From Novel Coronavirus 2019 at 21 Days: A Retrospective Cohort Study. 2/1/21. Harrison RF. Crit Care Explor.
Of 1027 patients 60 years old or older admitted for COVID-19, the 28 who were on warfarin upon admission did not have a significantly different mortality at 28 days than the 894 patients who were on no anticoagulation. However, the 104 patients taking a direct oral anticoagulant had improved mortality (14.4% vs 23.8%; odds ratio, 0.57) prior to adjustment and after controlling for age, gender, and comorbidities. They had a mortality odds ratio of 0.44 when compared to patients on no oral anticoagulant on admission. No statistical difference was noted between the groups in the prevalence of bleeding events. The authors recommend an RCT to better evaluate this possible effect. - Assessment of Maternal and Neonatal Cord Blood SARS-CoV-2 Antibodies and Placental Transfer Ratios. 1/29/21. Flannery DD. JAMA Pediatr.
This data rich article is from a single institution describing a study in which 6% of 1714 women became infected with COVID-19 during pregnancy. This multi-ethnic/race study revealed that of the 83 women infected, 72 (87%) passed igG antibodies to the fetus offering neonatal protection from infection.
SAB Comment: Maternal infections during pregnancy (HIV) can alter IgG transfer to the fetus. Reference: Immunohorizons. 2018 Jan 1; 2(1): 14–25. - A rapid review of the pathoetiology, presentation, and management of delirium in adults with COVID-19. 12/25/20. Hawkins M. J Psychosomatic Res.
In this in-depth literature review of delirium in COVID-19, the authors initially identified 10,000 publications and after removing duplicates and screening abstracts, 229 studies were included in the review. This review serves as a source of reference for intensivists dealing with various aspects of diagnosing and treating delirium. After reviewing current information on prevalence, symptoms and etiology, prevention and management are highlighted in a summarizing table. In the absence of randomized clinical trials on this topic, the discussion is limited to reporting diverse empirical management with and without pharmacological intervention, stressing the fact that delirium can be a core symptom at presentation and may be under-recognized and under-diagnosed.
Newsletter Issue 57, February 3, 2021:
- Effect of Discontinuing vs Continuing Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers on Days Alive and Out of the Hospital in Patients Admitted With COVID-19: A Randomized Clinical Trial. 1/19/21. Lopes RD. JAMA.
This article discussed the BRACE CORONA trial, a registry-based open label clinical trial of 659 participants admitted to 29 Brazilian hospitals with mild to moderate COVID-19 disease. The results corroborated that discontinuing ACEI or ARB therapy for 30 days did not affect the number of days alive and out of the hospital in patients hospitalized with mild to moderate COVID-19.
SAB Comment: This trial is characterized by a single-country design and a notably younger (mean age 55 years) patient population with less comorbid disease (33% with diabetes; 5% with cardiovascular diseases) than the REPLACE COVID trial and had fewer total deaths despite the larger sample size (3% mortality in the BRACE CORONA trial vs 14% mortality in the REPLACE COVID trial). - Information on Respiratory Protection
For readers interested in respiratory protection including N95 filtering facepiece respirators (FFR), our special sections at the end of the newsletter and on our website have been updated with 2 new publications, including summaries and direct links, and a spreadsheet of testing information of hundreds of new FFR manufactured in various countries, performed by NIOSH and ECRI, a health quality institute. - Vaccine Updates
- Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. 12/31/20. Baden LR. NEJM.
This phase 3 randomized, observer-blinded, placebo-controlled trial was conducted at 99 centers across the US. Persons at high risk for SARS-CoV-2 infection or its complications (with locations or circumstances that put them at an appreciable risk of SARS-CoV-2 infection, a high risk of severe COVID-19, or both) were randomly assigned in a 1:1 ratio to receive two intramuscular injections of mRNA-1273 (Moderna) (100 μg) or placebo 28 days apart. Site-selection and enrollment processes were adjusted to increase the number of persons from racial and ethnic minorities in the trial. The mRNA-1273 vaccine showed 94.1% efficacy at preventing COVID-19 illness, including severe disease. All the severe COVID-19 cases were in the placebo group. Aside from transient local and systemic reactions, no safety concerns were identified. - Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. 12/31/20. Polack FP. NEJM.
In this ongoing multinational, placebo-controlled, observer-blinded, pivotal efficacy trial, the authors randomly assigned persons 16 years of age or older in a 1:1 ratio to receive two doses, 21 days apart, of either placebo or the BNT162b2 (BioNTech-Pfizer) vaccine candidate. Forty-three thousand four hundred forty-eight (43,448) individuals received injections. Eight patients who received the vaccine developed COVID-19 compared to 162 patients who received placebo, i.e., the vaccine was 95% effective. None of the eight vaccinated patients who developed COVID-19 required hospitalization. The safety profile included short-term, mild-to-moderate pain at the injection site, fatigue, and headache. Although the vaccine can be stored for up to 5 days at standard refrigerator temperatures once ready for use, very cold temperatures are required for shipping and longer storage.
- Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. 12/31/20. Baden LR. NEJM.
Newsletter Issue 56, February 1, 2021:
- Intermediate-dose anticoagulation, aspirin, and in-hospital mortality in COVID-19: a propensity score-matched analysis. 1/21/21. Meizlish ML. Am J Hematol.
This article describes a complex scientific analysis of 2785 hospitalized COVID-19 patients advocating for the daily use of 0.4-0.7 mg/kg enoxaparin, which is considered an intermediate dose, and 81 mg daily aspirin as the means to achieve the lowest in-hospital mortality from COVID-19 from thromboembolic cause. The Rothman Index (RI), a computer based and complex in itself calculation was used as a means of defining study groups. - Prevalence of Thrombotic Complications in ICU-Treated Patients With Coronavirus Disease 2019 Detected With Systematic CT Scanning. 1/20/21. Mirsadraee S. Crit Care Med.
72 ICU patients with COVID-19 underwent CT of the chest, abdomen and pelvis on admission to a tertiary care cardiorespiratory ICU. 35 were on ECMO. 58% had thrombosis, most commonly in the pulmonary arteries (47%). Thrombosis was not correlated with biomarkers, however mortality was significantly greater (33% vs 10%; p = 0.022). All but one patient was receiving at least prophylactic doses of anticoagulation. The authors suggest the routine use of CT scanning of the chest, abdomen and pelvis for extremely ill COVID-19 patients.
SAB Comment: These authors have a CT scanner in proximity to their ICU. - Occurrence of Invasive Pulmonary Fungal Infections in Severe COVID-19 Patients Admitted to the ICU. 1/22/21. Fekkar A. Am J Respir Crit Care Med.
A careful clinical review of 145 severely ill (72 on ECMO), intubated COVID-19 patients in March and April 2020 where suspicion of ventilator acquired pneumonia led to evaluation for invasive fungal infection. 475 respiratory samples were submitted to direct examination/culture, galactomannan index determination, and PCR targeting. In addition, 532 sera were tested for galactomannan, beta-D-glucan, and/or A. fumigatus PCR. Only 4.8% of these patients were felt to have invasive fungal disease while another 17.2% were considered to have clinically irrelevant colonization. Most were not being treated with steroids. Patients on long-term high-dose steroids and recipients of solid organ transplants were at highest risk. - Residual symptoms and lower lung function in patients recovering from SARS-CoV-2 infection. 1/22/21. Trinkmann F. Eur Respir J.
This is a study of persistent symptoms and PFT abnormalities 2 months after PCR-proven infection in 246 consecutive initially symptomatic patients (median age 48). Only 9% required hospital admission or received specific therapy, and only 2 of the 22 hospitalized patients required ICU treatment. However, at follow up, 46% still had 1-3 symptoms, particularly those initially more symptomatic. Symptom frequencies at presentation vs. 2 month follow-up were olfactory loss (66% vs. 4%), cough (65% vs 14%), fever (59% vs <1%), dyspnea (31% vs 32%), sore throat (26% vs <1%), rhinitis (25% vs 1%), headache (16% vs <1%), and fatigue (6% vs 1%). Mild spirometry and DLCO reductions were found in persistently symptomatic patients. - Core Outcome Measures for Trials in People With Coronavirus Disease 2019: Respiratory Failure, Multiorgan Failure, Shortness of Breath, and Recovery. 1/5/21. Tong A. Crit Care Med.
This is a multinational effort of 130 panel participants to establish standardized core outcome measures for research trials involving COVID-19 patients. Definitions and scoring criteria were established for respiratory failure, multiple organ failure, shortness of breath and recovery. If adopted in all trials, these relevant, meaningful, and feasible to implement sets of core outcome measures can strengthen the evidence basis for decision-making and can reduce wasted research efforts. - Pediatric Eye Injuries by Hydroalcoholic Gel in the Context of the Coronavirus Disease 2019 Pandemic. 1/21/21. Martin GC. JAMA Ophthalmol.
This is a brief report describing a 7-fold increase in pediatric eye injuries during the COVID-19 pandemic resulting from increased prevalence and use of alcohol-based hand sanitizer. The authors urge for the safe use of hand sanitizers and to warn parents and caregivers of the danger presented to children.
Newsletter Issue 55, January 27, 2021:
- Development and validation of the ISARIC 4C Deterioration model for adults hospitalised with COVID-19: a prospective cohort study. 1/11/21. Gupta R. Lancet Resp Med.
ISARIC4C is a global initiative with the purpose of preventing illness and death from infectious disease outbreaks. This article presents information on development and validation of a multivariable logistic regression model for in-hospital clinical deterioration (defined as any requirement of ventilatory support or critical care, or death) among consecutively hospitalized adults with suspected or confirmed COVID-19 prospectively recruited to ISARIC4C study across 260 hospitals in England, Scotland, and Wales. The authors contend that the 4C Deterioration model, designed to be used on admission, has strong potential for clinical utility and generalizability to predict clinical deterioration and inform decision-making among adults hospitalized with COVID-19. The Mortality and Deterioration calculator can be accessed with the following link: isaric4c.net/risk/.
SAB Comment: While the SAB does not endorse management strategies or interventions, its members believe this manuscript and accompanying calculator to evaluate risk of disease progression or death MAY be useful in supplementing case management decisions. - Improving clinical management of COVID-19: the role of prediction models. 1/11/21. Wynants L. Lancet Resp Med.
This is an editorial indicating that the main clinical advantage of the ISARIC4C predictive model is that required patient specific data is available from daily routine care and may help inform stratification of patients on the basis of clinical severity. In combination, the 4C Deterioration and Mortality models could be utilized in creating an evidence-based clinical pathway for patients with COVID-19. Validated predictive models may improve clinical management and resource utilization.
Newsletter Issue 54, January 25, 2021:
- Renin-angiotensin system inhibitors in hospitalised patients with COVID-19. 1/10/21. Williams B. Lancet Respir Med.
This editorial provides a commentary on the ACEI/ARB controversy. While highlighting the REPLACE COVID trial which examined the impact of continuing or withdrawing chronic ACEIs or ARB treatment in 152 patients hospitalized with COVID-19 across 20 international centers which resulted in no difference in outcome, it stresses the global collaboration, scale and speed with which investigators conducted observational cohort studies with similar results which made this small RCT’s results convincing. In addition, the authors refer to the larger BRACE CORONA RCT with identical yet unpublished results and refer to recent literature showing that there is no increase in ACE2 expression caused by ACEIs and ARBs in pulmonary or renal tissue. - The Association of Low Molecular Weight Heparin Use and In-hospital Mortality Among Patients Hospitalized with COVID-19. 1/4/21. Shen L. Cardiovasc Drugs Ther.
This paper examines 525 COVID-19 hospitalized patients from Wuhan. Twenty-three percent were treated with low molecular weight heparin (LMWH). These patients were likely to be older, have more co-morbidities and had more severe COVID-19 parameters. Compared with non-LMWH group, LMWH group had a higher unadjusted in-hospital mortality rate (21.70% vs. 11.10%; p = 0.004), but a lower adjusted mortality risk (adjusted odds ratio [OR], 0.20; 95% CI, 0.09–0.46). These retrospective data suggest that LMWH use was associated with lower all-cause in-hospital mortality. The survival benefit was particularly significant among more severely ill patients.
SAB Comment: This retrospective study suggests benefits of LMWH on mortality and contributes to the ongoing debates about the use of anticoagulants in these patients. This further highlights the need for the upcoming RCTs. - Venous thromboembolism and major bleeding in patients with COVID-19: A nationwide population-based cohort study. 1/5/21. Dalager-Pedersen M. Clin Infect Dis.
This review from 6 Danish hospitals examines 30-day VTE and bleeding risks in 9,460 PCR+ patients for SARS-CoV-2, 226,510 SARS-CoV-2 negative patients and 16,281 patients with influenza. One thousand five hundred and forty of the COVID-19 patients were hospitalized. Overall 30-day risk for VTE was 0.4% (40/9,460) among COVID-19 positive patients compared with 0.3% (649/226,510) for COVID-19 negative patients and 1.0% (158/16,281) among influenza patients. Among hospitalized patients, risks for VTE were 1.5% (23/1,540) in COVID-19 positive patients compared with 1.8% (483/26,131) in COVID-19 negative patients and 1.5% (147/9,599) in hospitalized influenza patients. No differences were noted in major bleeding events. In this nationwide survey, the data demonstrate a low rate of VTE and bleeding for outpatients with SARS-CoV-2. - Thromboembolic complications in critically ill COVID-19 patients are associated with impaired fibrinolysis. 12/8/20. Kruse JM. Crit Care.
While this review only has 40 ICU COVID-19 patients, the data suggest that a severe decrease in clot breakdown is a cause of the coagulopathy associated with COVID-19. Maximum lysis, especially following stimulation of the extrinsic coagulation system using rotational thromboelastometry (ROTEM), was inversely associated with an enhanced risk of thromboembolic complications. Combining values for maximum lysis with D-dimer concentrations revealed high sensitivity and specificity of thromboembolic risk prediction (area under curve of 0.92). - Neutralizing antibody titres in SARS-CoV-2 infections. 1/4/21. Lau E. Nature Communications.
SARS-CoV-2 infection elicits effective neutralizing antibody titers in most individuals. Using plaque reduction neutralization (PRNT) assays, a “gold-standard,” kinetics of virus neutralizing antibody responses were examined from a cohort of 195 infections collected days 0 to 209 after symptom onset. Of 115 sera collected ≥61 days after onset of illness tested, 99.1% remained seropositive for both 90% (PRNT90) and 50% (PRNT50) neutralization endpoints. Investigators estimated it takes at least 372, 416 and 133 days for PRNT50 titers to drop to the detection limit for severe, mild, and asymptomatic patients, respectively. Results were uninfluenced by age or corticosteroid use. - Circuits between infected macrophages and T cells in SARS-CoV-2 pneumonia. 1/11/21. Grant RA. Nature.
In this basic science article, using flow cytometry and transcriptomic profiling these investigators compared bronchoalveolar lavage (BAL) samples from 88 patients with SARS-CoV-2 respiratory failure to 211 patients with non-SARS-CoV-2 failure. In 10 SARS-CoV-2 BALs they analyzed single-cell RNA-seq. In SARS-CoV-2, the alveolar space was enriched in T cells (CD4+ and CD8+) and monocytes; only 31% had neutrophilia. Transcriptomes suggested that SARS-CoV-2 directly infects alveolar macrophages (AM), which produce T-cell chemo-attractants. T-cells then produce interferon-gamma. Feedback to AM promotes further T-cell activation. In contrast to non-SARS-CoV-2, SARS-CoV-2 causes a slowly unfolding, spatially limited alveolitis. Infected AM and T cells form a positive feedback circuit.
Newsletter Issue 53, January 20, 2021:
- Stability of SARS-CoV-2 on critical personal protective equipment. 1/13/21. Kasloff SB. Nature Scientific Reports.
Persistence of viable virus was measured on eight PPE materials. Viable SARS-CoV-2 persisted for 21 days on plastic, 14 days on stainless steel, 7 days on nitrile gloves and 4 days on chemical resistant gloves, though at significantly reduced levels compared to the initial inoculum. Viable SARS-CoV-2 was nearly undetectable, but could still be recovered from N-95 and N-100 materials for up to 21 days. On 100% cotton, the virus underwent rapid degradation and was not detectable within 24 hours. These findings underline the importance of appropriate handling of contaminated PPE and a potential advantage of cotton.
SAB Comment: Many of the results in this study differ from other often-quoted reports. This is not surprising as experimental conditions including contaminating load, medium, ambient temperature and humidity have a large influence on the time viruses remain viable and vary among studies. - Persistent Post-COVID-19 Inflammatory Interstitial Lung Disease: An Observational Study of Corticosteroid Treatment. 1/12/21. Myall KJ. Ann Am Thorac Soc.
This is a well-written narrative following 837 COVID-19 patients seen between February and May 2020 in metropolitan London, UK hospitals. Four weeks after discharge, 39% had not returned to baseline and underwent further study. Thirty patients with persistent respiratory symptoms and interstitial lung disease received an initial maximal dose of prednisolone 0.5mg/kg with rapid weaning over a 3-week period which improved diffusion capacity by 31.6% and FVC by 9.6% which resulted in symptomatic and radiological improvement. This preliminary data requires further study into the natural history and potential treatment for patients with persistent inflammatory interstitial lung disease following SARS-CoV2 infection. - Ventilator-associated pneumonia in critically ill patients with COVID-19. 1/12/21. Maes M. Crit Care.
This retrospective observational study from the UK studied ventilator-associated pneumonia (VAP) in mechanically ventilated COVID-19 (n=81) and non-COVID-19 (n=144) patients at a single hospital. All patients were studied between March and August 2020, and VAP was defined by the European Center for Disease Control using clinical and microbiological criteria. COVID-19 was associated with an increased risk of VAP (28 per 1,000 ventilator days) compared with non-COVID-19 patients (13 per 1,000 ventilator days). Although the distribution of organisms causing VAP was similar between the two groups, aspergillosis was only found in COVID-19 patients (n=3, none on steroids), though one patient without COVID-19 was borderline positive and met clinical criteria. - Lung ultrasound score predicts outcomes in COVID-19 patients admitted to the emergency department. 1/11/21. de Alencar JCG. Ann Intensive Care.
Lung ultrasound (LUS) was performed in the emergency department (ED) on 180 patients who were PCR positive for COVID-19. The protocol involved the examination of 12 lung regions, was performed at bedside by experienced ED physicians, and typically required five minutes. LUS scores correlated with findings from chest commuted tomography (CT) (when performed) and predicted the estimated extent of parenchymal involvement, death, endotracheal intubation, and ICU admission. The authors believe that LUS is more sensitive than chest radiography, requires less resource and infection risk than CT, and could be used as an effective evaluation tool, particularly in resource-constrained settings. - Duration and key determinants of infectious virus shedding in hospitalized patients with coronavirus disease-2019 (COVID-19). 1/12/21. van Kampen JJA. Nat Commun.
The CDC currently uses a minimum disease duration of 10 days in their symptom-based strategy as the statistically estimated likelihood of recovering a replication-competent virus approaches zero after ten days of symptoms. In a study of 129 hospitalized patients, duration and key determinants of infectious SARS-CoV-2 shedding in patients with severe and critical COVID-19 was assessed. Median time for infectious virus shedding was 8 days post-symptom onset; ≤5% probability for isolating infectious SARS-CoV-2 when duration of symptoms was ≥ 15.2 days. Median viral load was significantly higher in culture + samples than culture – samples. Probability of isolating infectious virus was < 5% when neutralizing antibody titer was 1:80 or higher. Detection of subgenomic RNAs outlasted detection of infectious virus. Based on their findings, a longer disease duration could be considered for severely-ill patients.
Newsletter Issue 52, January 18, 2021:
- COVID-19, Personal Protective Equipment, and Human Performance. 1/6/21. Ruskin KJ. Anesthesiology.
This article addresses the issue of the varieties of PPE worn by healthcare workers. The authors discuss how various varieties of PPE may cause increased work of breathing, reduced field of vision, communication mishaps, thermoregulation derangements, limitations of physical dexterity and mental, physical, and psychological fatigue and stress which lead to decreased human performance. These effects are not individual weaknesses. Here the authors suggest some helpful remedies to address the physiologic and psychologic challenges imposed by non-standardized PPE. There is a need for a new, standardized, integrated design for PPE to improve the safety of patients and healthcare workers. - SARS-CoV-2 Transmission From People Without COVID-19 Symptoms. 1/7/21. Johansson MA. JAMA Netw Open.
A decision analytical model was used including multiple scenarios for the infectious period and the proportion of transmission from individuals who never have COVID-19 symptoms. Baseline assumptions were taken from meta-analyses and included an incubation period of a median of 5 days. In the various analyses peak infectiousness was varied between 3 and 7 days. Under a broad range of values for each of these assumptions, at least 50% of new SARS-CoV-2 infections were estimated to have originated from exposure to individuals who were asymptomatic at the time of transmission (combining those who never develop symptoms with those who are pre-symptomatic).
SAB Comment: This highlights the importance of mask-wearing and social distancing even as vaccines are rolled out. - Facial Pressure Injuries from Prone Positioning in the COVID-19 Era. 1/3/21. Shearer SC. Laryngoscope.
This study highlights the high frequency (48%) of facial pressure injuries associated with intubated COVID-19 patients placed in the prone position at a single US institution. Most of these patients were continuously in the prone position. Of 143 intubated ICU patients proned for an average of 123 hours, cheek and ear injuries accounted for the majority of damage, with the likelihood of injury increasing as proning times increased. A particular problem seemed to be pressure caused by commercial endotracheal tube fasteners. Suggestions for reducing these injuries are made. The study did not address injuries to the eye or elsewhere on the body. - Impact of cardiovascular disease and risk factors on fatal outcomes in patients with COVID-19 according to age: a systematic review and meta-analysis. 12/18/20. Bae S. Heart.
This is a retrospective meta-analysis to investigate the impact of cardiovascular disease (CVD) and associated risk factors (hypertension, diabetes) on age-related mortality in COVID-19 patients. Fifty-one studies, including 48,171 patients were included, along with PRISMA diagrams and tables. Unsurprisingly, CVD, hypertension and diabetes increased mortality across all groups. However, when present in younger ages, the odds ratio of mortality compared with same age patients without the risk factors was disproportionately higher than the same age ratio in the elderly. While young patients had lower prevalence rates of cardiovascular comorbidities than elderly patients, relative risk of fatal outcome in young patients with hypertension, diabetes and CVD was higher than in elderly patients. - Early corticosteroids are associated with lower mortality in critically ill patients with COVID-19: a cohort study. 1/5/21. Monedero P. Crit Care.
This is a prospective, multicenter, observational, cohort study in 882 critically ill adult patients with COVID-19 admitted to 36 critical care units in Spain. Beginning in early March to the end of June 2020, patients receiving corticosteroids within 48 hours of ICU admission had a lower mortality compared to those receiving steroids later (30 vs. 40% – HR 0.71) or not at all. Patients treated early did better overall with shorter ICU stays, fewer ventilator days and a lower incidence of organ dysfunction. Higher dosages were found to be more effective. Corticosteroid administration occurred on average 12 days after symptom onset. The authors recommend corticosteroids as early as day 7 provided inflammatory markers are elevated.
Newsletter Issue 51, January 13, 2021:
- Antibody Status and Incidence of SARS-CoV-2 Infection in Health Care Workers. 12/28/20. Lumley SF. N Engl J Med.
This is an original article from 4 Oxford University Hospitals that followed its employees for SARS-CoV-2 infection. Testing was performed every 2 weeks or if symptomatic. 10% of 12,541 staff tested positive from March through November 2020. Polymerase chain reaction assays of both anti-spike IgG and anti-nucleocapsid IgG demonstrated that healthcare workers who tested positive suffered mild disease and were afforded immunity for the length of the study, 31 weeks. - 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. 1/8/21. Huang C. Lancet.
175-199 days after symptom onset, 1733 of 2469 discharged Wuhan COVID-19 patients (median age 57) completed questionnaires to evaluate symptoms and quality of life along with physical examinations, a 6-min walking test, and blood tests. Reduced 6-min walk, fatigue, pulmonary abnormalities, and anxiety or depression were prevalent. 73% of men and 81% of women reported at least one symptom (76% overall). Most common were fatigue or muscle weakness (63%), sleep difficulties (26%), and anxiety or depression (23%). Symptoms were positively correlated with previous COVID-19 illness severity.
SAB Comment: Many with mobility or neurologic issues were excluded, therefore accurate percentages may be higher. - Early High-Titer Plasma Therapy to Prevent Severe Covid-19 in Older Adults. 1/6/21. Libster R. N Engl J Med.
This article describes an Argentine randomized, double-blind, placebo-controlled trial of convalescent plasma (CP) with IgG titers >1:1000 against SARS-CoV-2 within 72 hours following the onset of mild COVID-19. CP reduced disease progression in adult patients older than 75 years or 65-74 years old with co-morbidities. Severe respiratory disease developed in 13/80 patients (16%) who received 250 ml of CP and 25/80 (31%) who received 250 ml normal saline (relative risk, 0.52). Benefit was more frequent following units with higher IgG titers, indicating a dose-dependent effect. Deaths were 2/80 in the CP group vs. 4/80 in the placebo group. - Hypercoagulopathy in Severe COVID-19: Implications for Acute Care. 12/28/20. Waite AAC. Thromb Haemost.
This is a review of the coagulopathy associated with COVID-19. This is a good summary of the early literature up to current papers. It includes clinically relevant data and upcoming trials. This paper is a useful update. - Incidence of symptomatic, image-confirmed venous thromboembolism following hospitalization for COVID-19 with 90-day follow-up. 12/22/20. Salisbury R. Blood Adv.
This paper presents new data examining venous thromboembolism (VTE) after discharge for patients with COVID-19. 303 patients were treated at Oxford University Hospitals. 5.9% were diagnosed with VTE in the hospital. Data were collected in 205 patients post discharge up to 90 days or until death if earlier. Based upon the national registry, 2.6% had VTE, all pulmonary emboli. The authors suggest that this needs to be further investigated as there may be a beneficial role for post-discharge anticoagulation in some patients.
Newsletter Issue 50, January 11, 2021:
- Adaptation of an Obstetric Anesthesia Service for the Severe Acute Respiratory Syndrome Coronavirus-2 Pandemic: Description of Checklists, Workflows, and Development Tools. 12/14/20. Li Y. Anesth Analg.
In this pragmatic report from Boston, authors share their revised workflows and checklists for all aspects of obstetric anesthesia care in the COVID-19 era. A cyclical improvement methodology was used to design each new workflow. Challenges include simultaneous care of infected patients alongside noninfected patients, inherent uncertainties regarding the course of labor and delivery, and need for coordination with other departments such as neonatology. Authors developed independent workflows and procedure-specific material kits that may be used alone or in sequence without extensive staff training to facilitate care and conserve resources. - Update to living systematic review on drug treatments for covid-19. 12/18/20. Siemieniuk RAC. BMJ.
First 6-month follow-up and therefore “living” review of randomized trials examining COVID-19 treatment options were discussed, specifically their comparative effectiveness that have not been tested head-to-head. Leading off with a uniquely designed interactive infographic diagram (available on the web but not featured on the PDF download) that links therapeutics to 11 different outcomes, the review tabulates and discusses results and allows for a quick search as well as in-depth sourcing using extensive tables and bibliographic links sorted by outcome modality. This encyclopedic review originated from 20 institutions in 13 countries, including 3 in China, and in its conclusions highlights the beneficial effect of glucocorticoids on mortality and mechanical ventilation, while questioning other drugs, including tocilizumab and remdesivir. - A Neutralizing Monoclonal Antibody for Hospitalized Patients with Covid-19. 12/28/20. ACTIV-3/TICO LY-CoV555 Study Group. N Engl J Med.
The antiviral drug remdesivir has been shown to decrease the time to recovery in hospitalized patients with COVID-19. However, the preliminary results of a study involving a single infusion of the neutralizing monoclonal antibody LY-CoV555 when co-administered with remdesivir, did not demonstrate efficacy, among hospitalized patients (sample size 300) who had COVID-19 without end-organ failure, measured at day 5 vs those who received placebo. LY-CoV555 met the pre-specified criteria for futility and further enrollment was stopped. - Factors Associated with Positive SARS-CoV-2 Test Results in Outpatient Health Facilities and Emergency Departments Among Children and Adolescents Aged <18 Years – Mississippi, September-November 2020. 12/17/20. Hobbs CV. MMWR Morb Mortal Wkly Rep.
This investigation included children and adolescents younger than 18 years who received RT-PCR testing for presence of SARS-CoV-2 in nasopharyngeal swab specimens at outpatient testing health care centers or the ED during September 1–November 5, 2020. Of 397 participants, children and adolescents who received positive test results for SARS-CoV-2 were more likely than were similarly aged participants who had negative test results to have had close contact with persons with COVID-19. Exposure was attributed to gatherings with persons outside the household, and a lack of consistent mask use in school. However, attending school or childcare was not associated with receiving positive SARS-CoV-2 test results. - SARS-CoV-2 Variant – United Kingdom of Great Britain and Northern Ireland. 12/21/20. WHO.
UK scientists sequenced a SARS-CoV-2 variant (VUI 202012/01) now representing >50% of isolates in South East England. The variant shows 14 mutations resulting in amino acid changes and three deletions. Significant mutations in the receptor binding domain are N501Y and P681H. A deletion at position 69/70 affects the Spike (S)-gene. The variant increases transmissibility between 40-70%, adding 0.4 to R0 bringing it to 1.5-1.7. Investigations are ongoing to determine if this variant will change symptom severity, antibody responses or vaccine efficacy. Most PCRs target multiple sequences and therefore the impact of the variant on diagnostics is not anticipated to be significant.
SAB Comment: In order to understand the epidemiology of any variant, widespread and frequent genetic sequencing of viral testing samples is needed. Currently, the US lags far behind the UK in this regard, sequencing ~1% of samples vs. >10% in the UK. Therefore, relatively little is known about the spread of the “UK variant” in the US. - Genetic mechanisms of critical illness in Covid-19. 12/11/20. Pairo-Castineira E. Nature.
Oriented towards research, this genome-wide association study (GWAS) examined 2,244 critical COVID-19 patients in 208 UK ICUs to uncover gene variants that are severity markers and potential treatment targets. GWAS findings implicated antiviral restriction enzyme activators (OAS1/OAS2/OAS3), high tyrosine kinase-2 (TYK2), dipeptidyl peptidase- 9 (DPP9) and low interferon receptor gene IFNAR2. Mendelian randomization techniques implicated as “causal” low IFNAR2 and high TYK2 expression. Lung tissue transcriptome-wide association implicated high monocyte/macrophage chemotactic receptor CCR2. These gene alterations implicating early anti-viral defense (IFNAR2, OAS) and late inflammation (DPP9, TYK2, CCR2) can be evaluated in clinical trials using licensed drugs (interferons, JAK inhibitors, CCR2 inhibitors, etc.).
Newsletter Issue 49, January 6, 2021:
- Variation in US Hospital Mortality Rates for Patients Admitted With COVID-19 During the First 6 Months of the Pandemic. 12/22/20. Asch DA. JAMA Intern Med.
This cohort study from a US-managed health company evaluated outcomes for 38,517 adults with COVID-19 admitted to 955 US hospitals during two time periods (January to April and May to June). The primary outcome was the hospitals’ risk-standardized event rate (RSER) of 30-day in-hospital mortality or referral to hospice, adjusted for patient-level characteristics. RSERs declined from 16.6% to 9.3%. Individual hospitals did better when the prevalence of COVID-19 in their surrounding communities was lower. The article speculates on possible causes for this improvement, including fewer overwhelmed hospitals, improved knowledge and medical care, and possibly smaller infective inoculums as mask-wearing became more common. - Factors Associated With Severe SARS-CoV-2 Infection. 12/16/20. Ouldali N. Pediatrics.
This 60-hospital French national study used an established meningitis surveillance network to study demographics of COVID-19 pediatric patients. Data collection was from February 15-June 1, with 397 children and included an estimated 38.5% of the total cases in France. The primary outcome was the proportion of patients with disease progression, and secondary outcomes were defined by age groups. The median age was 16 months. Three percent of children (4/135) <90 days old developed severe disease. MIS-C increased with age. There was severe disease overall in 11% (23/306). Of the 6 mortalities only one was entirely due to COVID-19. Findings suggested that the rate of severe forms was the lowest in very young children and was the highest for children ≥ 10 years. - Corticosteroids for Patients With Coronavirus Disease 2019 (COVID-19) With Different Disease Severity: A Meta-Analysis of Randomized Clinical Trials. 12/10/20. Pasin L. J Cardiothorac Vasc Anesth.
In this meta-analysis of 5 studies involving treatment with steroids of 7,692 COVID patients, the authors note that the effect of corticosteroids therapy on survival with COVID patient varies with different respiratory support. The use of corticosteroids may be detrimental to patients who do not require oxygen support (NNH/number needed to harm=29) and increases mortality. Its effectiveness for mechanically ventilated patients was (NNT/number needed to treat=19). The majority of COVID-19 patients not requiring oxygen/mechanical ventilation will have a better survival benefit without steroids. - Corticosteroid use in COVID-19 patients: a systematic review and meta-analysis on clinical outcomes. 12/15/20. van Paassen J. Crit Care.
This well-conducted systematic review and meta-analysis to evaluate safety and effectiveness of corticosteroids in COVID-19 included 44 studies and 20,197 patients collected between December 1, 2019 and October 1, 2020. Primary outcomes were short-term mortality and viral clearance (based on RT-PCR in respiratory specimens). Secondary outcomes were need for mechanical ventilation, other oxygen therapy, length of hospital stay and secondary infections. Non-peer reviewed and pre-published manuscripts were excluded from analysis. Findings from observational studies and RCTs confirm beneficial effect of corticosteroids on short-term mortality and reduction in mechanical ventilation. A possible signal of delayed viral clearance and an increase in secondary infections was noted. Optimal timing, dose and duration of corticosteroids, in relation to safety, remain subjects for further investigation. - COVID-19 Convalescent Plasma Treatment of Moderate and Severe Cases of SARS-CoV-2 Infection: A Multicenter Interventional Study. 12/7/20. Alsharidah S. Int J Infect Dis.
SAB Comment: This is a non-randomized observational study from Kuwait. Research is needed to determine patient groups that benefit.
One hundred and thirty-five patients with moderate-severe COVID-19 disease who received 2 units of convalescent plasma (CP) within 3 days of hospital admission had an earlier and higher rate of clinical improvement compared with 233 control patients. Moderate disease was found in 86.5% of CP group (n=89) who had a time to clinical improvement of 7 days versus 68% of controls with time to clinical improvement of 8 days (p=0·006). Severe disease was found in 61% of CP group (n=46) with time to clinical improvement of 7 days vs. 35% of controls with time to clinical improvement of 15.5 days (p=0·003). Overall 30-day mortality was 18% CP group vs. 39% controls. Moderate disease patients had a significantly lower mortality following CP (11% vs. 30%, p= 0.001). - Masking the 6 Minutes-Walking-Test in the COVID-19 Era. 12/14/20. Salles-Rojas A. Ann Am Thorac Soc.
A small study of 77 COVID-19 pneumonia survivors who each performed the 6-Minute Walking Test twice, once with a surgical or an N-95 mask and once without a mask. No differences were observed between wearing or not wearing a mask in the meters walked, SpO2, HR, dyspnea or fatigue.
Newsletter Issue 48, January 4, 2021:
-
A Year in Review and Brief Survey
The IARS COVID-19 Resource Newsletter is a unique resource for anesthesiologists, intensivists, and other front-line healthcare providers. Since launch of the newsletter last March, the COVID-19 Scientific Advisory Board (SAB) has screened more than 85,000 peer-reviewed journal articles to identify the information most useful for our intended audience. More than 1,750 articles have undergone thorough review by the SAB members, and 641 have been summarized by the SAB for the 88 issues of the newsletter we published in 2020. Unlike other society newsletters, the IARS newsletter pulls content from dozens of peer-reviewed journals to identify the information deemed most useful for physicians on the front lines and summarizes the key takeaways.
We hope you are one of the thousands of physicians who are benefitting from this resource. We would appreciate you taking just a few minutes to complete a brief survey about our COVID-19 initiative. We also welcome your comments and questions at any time. Please direct them to Meghan Whitbeck, [email protected].
Thank you for your interest.
Sincerely,
The COVID-19 Scientific Advisory Board
International Anesthesia Research Society
