COVID-19 Resources
Following declarations from the US Department of Health and Human Services and World Health Organization ending the COVID-19 Public Health Emergency, the IARS COVID-19 Scientific Advisory Board (SAB) concluded its review of the scientific literature about SARS-CoV-2 in August. The SAB has reviewed more than 3,100 journal articles and published 1,076 article reviews over the past 42 months. It has been an enormous commitment from the SAB, and the IARS owes our dedicated physician volunteers a huge debt of gratitude for their unwavering participation in this initiative.
To search by keyword, select Ctrl + F on a PC and Command + F on a Mac. Then, enter keyword and Enter.
Disclaimer
The material on this website is provided for informational purposes and does not constitute medical advice. New knowledge is added daily and may change over time. Opinions expressed should not be construed as representing IARS policy or recommendations. References and links to third parties do not constitute an endorsement or warranty by IARS.
The material on this website is provided for informational purposes and does not constitute medical advice. New knowledge is added daily and may change over time. Opinions expressed should not be construed as representing IARS policy or recommendations. References and links to third parties do not constitute an endorsement or warranty by IARS.
May 19, 2023:
- COVID-19 Vaccine Safety First Year Findings in Adolescents. 4/21/2023. Hesse EM. Pediatrics.
This paper reports data obtained via v-safe (a smartphone-based reporting system for reactions to the vaccine) and VAERS (Vaccine Adverse Event Reporting System of the CDC and FDA) from 12-17 year old recipients of at least 1 dose of Pfizer mRNA vaccine between 12/14/2020 and 5/10/2022. The v-safe data comes from 172,032 participants, 30% of whom were vaccinated in May 2021. The most common reaction was pain at the injection site (60%). The VAERS data include 20,240 reported events, of which 92% were non-serious. Using 32,268,525 doses administered as the denominator, the authors include a table of the rate per million doses of adverse events of special interest including myocarditis in 17.7 per million, although the rate was 84 per million for the second dose in 16-17 year olds. - Expert consensus statement on venovenous extracorporeal membrane oxygenation ECMO for COVID-19 severe ARDS: an international Delphi study. 5/2/23. Rabie AA. Ann Intensive Care.
The modified Delphi technique was used by 22 international extracorporeal membrane oxygenation (ECMO) experts worldwide to reach consensus on 14 general statements based on the most recent findings of the evolving published research. These 14 statements were in four domains (clinical, operational, and logistic ECMO management and ethics) and were formulated to guide next-generation ECMO providers during future pandemic situations. Two example statements are, 1) “The duration of invasive mechanical ventilation (IMV) before considering ECMO should not be used as a primary determinant for ECMO candidacy,” and 2) “There is no validated evidence-based scoring system to predict the outcome for COVID-19 patients receiving ECMO. Therefore, available scoring systems previously used for non-COVID-19 patients should not be used for COVID-19 patients as a prognostic tool.” - Lessons Learned From a COVID-19 Dog Screening Pilot in California K-12 Schools. 4/24/2023. Glaser CA. JAMA Pediatr.
Research letter exploring the efficacy of a program using canine scent screening for COVID-19 in school children as a rapid non-invasive, low cost and environmentally responsible method compared to a resource intensive universal antigen testing program. Two dogs underwent 2-month laboratory scent training to detect volatile organic compounds associated with COVID-19 infections resulting in 95% sensitivity and specificity in detecting the virus. Combined antigen and field-testing of 1558 student in 27 schools between April and May 2022 resulted in 3897 combined screenings. Sniffing the feet and ankles of students standing 6 feet apart, the dogs detected 85 infections, missed 18 and signaled 383 false positives resulting in a sensitivity of 83% and a specificity of 90% with antigen testing used as backup. Field testing, while rapid and low impact, has limitations but canine scent testing for COVID-19 and other pathogens should be considered where large scale screening is needed and resources are limited.
SAB Comment: The authors acknowledge the fact that one of the limitations of this study was the low incidence of infections during the test period. Not addressed is the potential need for re-training of dogs as new variants of the virus appear, emitting different volatile compounds. - Prevalence and Characteristics Associated With Post-COVID-19 Condition Among Nonhospitalized Adolescents and Young Adults. 3/30/23. Selvakumar J. JAMA Netw Open.
This study calls into question the usefulness of the WHO definition of post-COVID condition (PCC). The goal of this prospective Norwegian study conducted during the Alpha-dominant wave was to define the prevalence and risk of post-COVID condition (PCC) in young outpatients, a little-studied population. “This cohort study included 382 SARS-CoV-2–positive individuals and a control group of 85 SARS-CoV-2–negative individuals aged 12 to 25 years who were assessed at the early convalescent stage and at 6-month follow-up.” At inclusion and follow-up participants underwent a clinical interview, complete physical exam, measurement of vital signs, spirometry, electrocardiogram (ECG) including heart rate variability, cognitive function tests, blood tests including viral antibodies, cytokines and inflammatory markers, and questionnaires. Results: “When applying the World Health Organization case definition of PCC, prevalence at 6 months was 49%, but was also comparably high (47%) in the control group. PCC was not associated with biological markers specific to viral infection, but with initial symptom severity and psychosocial factors.” The main risk factor for PCC was symptom severity at baseline (RR, 1.41), and correlated with personality traits. Female sex, low physical activity, recent negative life events and loneliness were also factors.
SAB Comment: Our group found this study of interest, despite weaknesses that include a relatively small control group, and a question of whether self-selection may have played a role, given the number of hours required for the two evaluations. Additionally, although neuropsychiatric factors may be implied, we note that thorough neuropsychiatric testing was not part of the study. On the other hand the design was prospective and testing relatively complete compared with many if not most studies of post-COVID conditions. We hope it comes to the attention of the WHO.
April 21, 2023:
- Association of Treatment With Nirmatrelvir and the Risk of Post-COVID-19 Condition. 3/23/23. Xie Y. JAMA Intern Med.
A Veterans Administration cohort study, consisting of 87% men, to examine whether nirmatrelvir in the acute phase of SARS-CoV-2 infection lowers the risk of post-COVID-19 condition (PCC) or long COVID, defined as persistence of symptoms beyond 90 days past the acute episode. The authors identified 35,717 veterans who had been treated in 2022 with nirmatrelvir within 5 days of a positive COVID-19 test and 246,076 controls who had not received any antiviral or antibody treatment and who had at least one risk factor for progression to severe COVID-19. Using inverse probability weighting, the authors determined that nirmatrelvir reduced the relative risk of developing 10 of 13 prespecified symptoms of PCC by 26% (relative risk, 0.74; 95% CI, 0.72-0.77) at 180 days. Nirmatrelvir was also associated with decreased risk of death (47%) and hospitalization (24%). These results were independent of vaccination status or prior infection or re-infection.
SAB Comment: This study strongly supports nirmatrelvir administration during acute COVID-19 for at risk patients to mitigate PCC. - Effects of remdesivir in patients hospitalised with COVID-19: a systematic review and individual patient data meta-analysis of randomised controlled trials. 2/24/2023. Amstutz A. Lancet Respir Med.
The authors found nine eligible RCTs, covering 10 480 patients recruited between Feb 6, 2020, and April 1, 2021. Overall, 28-day mortality was 12·5% of 5317 patients assigned to remdesivir and 14·1% of 5005 patients assigned to no remdesivir. Of patients who were ventilated or received high-flow oxygen, 28-day mortality was 30·0% for patients assigned to remdesivir compared with 28·5% for patients assigned to no remdesivir. Of patients who received no oxygen or low-flow oxygen, 28-day mortality was 9·1% for patients assigned to remdesivir compared with 11·2% for patients assigned to no remdesivir.
SAB comment: While the initial NIH ACTT-1 randomized controlled trial in early 2020 showed a significant benefit achieved with remdesivir infusion, many clinicians have since been unconvinced of its effectiveness in their patients. These pooled results (of largely unvaccinated patients and which don’t include patients with the Delta or Omicron strains) suggest that remdesivir may not be effective in saving lives in patients requiring intensive respiratory support but may be somewhat effective in patients with milder respiratory impairment. - Efficacy of awake prone positioning in patients with covid-19 related hypoxemic respiratory failure: systematic review and meta-analysis of randomized trials. 2/6/2023. Weatherald J. BMJ.
This is the most recent meta-analysis of awake prone positioning (APP) for COVID respiratory distress. Seventeen randomized trials involving 2,931 patients were reviewed. The results confirmed prior meta-analyses, showing that APP reduces the need for intubation with 18 being the number needed to prone to prevent an intubation. Mortality and other secondary endpoints showed no improvement or worsening with APP. The patients who seemed to benefit the most were those that tolerated longer daily periods of APP, were initially more hypoxemic, and required high flow nasal oxygen or non-invasive ventilation. - Maternal third dose of BNT162b2 mRNA vaccine and risk of infant COVID-19 hospitalization. 3/24/23. Lipschuetz M. Nat Med.
This is a retrospective analysis of all live-born infants delivered in Israel between 24 August 2021 and 15 March 2022 to estimate the effectiveness of the third maternal Pfizer COVID-19 booster dose versus the second dose against infant COVID-19-related hospitalizations. Among 48,868 live-born infants included in the analysis, rates of COVID-19 hospitalization during the first 4 months of life were 0.7%, 0.6% and 0.4% in the unvaccinated, two-dose and three-dose groups, respectively, supporting clinical and public health guidance for maternal booster vaccination to prevent infant COVID-19 hospitalization. - Post-acute sequelae after SARS-CoV-2 infection by viral variant and vaccination status: a multicenter cross-sectional study. 3/11/2023. Kahlert CR. Clin Infect Dis.
Investigators performed a cross-sectional analysis (May/June 2022) within a prospective multicenter Swiss healthcare worker (HCW) cohort (n=2,912) of predominantly young, healthy Caucasian females. Uninfected HCWs served as controls. Previous infection during pre-Omicron BA-1 variant waves (Wild-type n=283 + Alpha/Delta n=268) was the strongest risk factor for post-acute COVID symptoms >4 weeks after infection, and lasting more than 7 days, when compared with those infected during the Omicron BA.1 wave (n=963) or no infection (n=1056). Eighteen symptoms as well as indices of depression and anxiety were assessed. Long Covid was diagnosed in 17% of those infected during the initial wave, 10% of those during Alpha/Delta and 5% of those during Omicron BA-1. The number of acute infection symptoms also decreased and was associated with the number of PASC symptoms. Those infected during the Omicron BA.1 wave had no additional protective effect from prior vaccination although the proportion of those unvaccinated was only 9%. - Symptom and Viral Rebound in Untreated SARS-CoV-2 Infection. 2/21/23. Deo R. Ann Intern Med.
This retrospective analysis of 563 placebo recipients (enrolled in ACTIV-2/A5401 randomized control trials of treatments for mild/moderate COVID-19) sought to characterize symptom and viral rebound in an untreated population. Patients, enrolled between November 2020 and July 2021 (pre-Omicron), were mostly unvaccinated. Patients reported daily scores for 13 symptoms over 28 days, and nasal swab quantitative viral RNA measurements were taken for days 1-14, 21, and 28. Symptom rebound occurred in 26%, was brief, and was associated with female sex, high risk factors for severe COVID, short time since symptom onset, and higher symptom score at study onset. Viral rebound occurred in 31%, was also brief, was not associated with high risk for severe COVID, but was associated with a high viral load at study onset. Both symptom and viral rebound occurred in only 3% of subjects.
April 4, 2023:
- COVID-19 bacteremic co-infection is a major risk factor for mortality, ICU admission, and mechanical ventilation. 1/23/23. Patton MJ. Crit Care.
A multicenter (UAB and Ochsner-LSU Shreveport systems), demographically diverse, cohort study of adult community-acquired bacteremic co-infection to date is lacking. This retrospective study evaluated bacteremic co-infection using 48-h post-admission blood cultures. Groupings: (1) confirmed (positive blood cultures), (2) suspected (negative culture with 2 or more doses antimicrobials administered), and (3) no evidence (no blood cultures obtained). The primary outcomes were in-hospital mortality, ICU admission, and mechanical ventilation. Cohorts:13,781 COVID-19 inpatients (2020 to 2022). Results: confirmed (2.5%), suspected (46%), no evidence (51.5%). The comparison cohort: 99,170 pre-COVID inpatients (2010-2019). An easily available elevated neutrophil-to-lymphocyte ratio greater than or equal to 15 (+ or – steroids) within 48-h of admission was a key marker of co-infection. Co-infection posed the greatest risk for in-hospital mortality, ICU admission and mechanical ventilation. Co-infection mortality (24%) with COVID far exceeds pre-pandemic inpatients (5.9%) and is consistent across alpha, delta, and omicron. - COVID-19 Vaccine Effectiveness Against Omicron Infection and Hospitalization. 3/3/2023. Piché-Renaud PP. Pediatrics.
Canadian investigators examined Ontario databases for BNT162b2 vaccine effectiveness (VE) against Omicron in children ages 5-11 (6284 test-positive cases and 8389 test-negative controls). VE was estimated by time since the latest dose vs. unvaccinated children and evaluated VE by dosing intervals. Symptomatic infection declined 24% (2-4 weeks after a first dose) and 66% (1-4 weeks after 2 doses). An extended 8-week dosing interval was associated with higher effectiveness against symptomatic infection for the first 3 months after vaccination. Severe outcomes were reduced 94% (7 to 29 days after 2 doses) and declined to 57% after 120 days. Conclusion: 2 doses of BNT162b2 provide moderate protection against symptomatic Omicron within 4 months of vaccination and good protection against severe outcomes. Protection wanes faster for infection than severe outcomes. Longer dosing intervals confer higher protection against symptomatic infection, but by 90 days, protection decreases and becomes like shorter dosing intervals. VE vs Omicron declined faster vs. former variants.  Effectiveness of nirmatrelvir-ritonavir in preventing hospital admissions and deaths in people with COVID-19: a cohort study in a large US health-care system. 3/18/23. Lewnard JA. Lancet Infect Dis.
Effectiveness of nirmatrelvir-ritonavir in preventing hospital admissions and deaths in people with COVID-19: a cohort study in a large US health-care system. 3/18/23. Lewnard JA. Lancet Infect Dis.
This is an observational outpatient study using electronic record analysis of the Southern California Kaiser Permanente HCS to further determine the effectiveness of nirmatrelvir–ritonavir (Paxlovid) in preventing hospital admissions and death within 30 days of a positive SARS-CoV-2 PCR test. Between April and October 2022, cases were matched by vaccination history, comorbidities, health care seeking trends and BMI in addition to age, sex and clinical status and patients who were tested within 5 days of symptom onset were analyzed separately. A total of 7,274 Paxlovid recipients were matched with 125,152 non-recipients and demonstrated an overall effectiveness in preventing hospital admission of 54% which increased to 80% among a smaller cohort treated within 5 days of symptom onset and to 90% when Paxlovid was dispensed on the day of a positive PCR test result. The authors discuss the etiology of 7 recently published observational studies conducted in the USA, Israel and Hong Kong, where Paxlovid effectiveness ranged from 21-79% in outpatients, and conclude that in a setting with high levels of vaccine uptake, Paxlovid is highly effective, particularly when given early.- Real-world use of nirmatrelvir-ritonavir in outpatients with COVID-19 during the era of omicron variants including BA.4 and BA.5 in Colorado, USA: a retrospective cohort study. 2/13/23. Aggarwal NR. Lancet Infect Dis.
A retrospective cohort study examined the real-world effectiveness of nirmatrelvir–ritonavir among high-risk outpatients with COVID-19 during the Omicron BA.2 and BA.2.12.1 (from March 26 to June 18, 2022) and BA.4 and BA.5 (from June 19 to Aug 25, 2022) waves in Colorado, USA. After propensity-score matching, 7168 patients treated with nirmatrelvir–ritonavir and 9,361 untreated controls were included for analysis. Outpatient use of nirmatrelvir–ritonavir reduced the odds of 28-day all-cause hospitalization from 1.4 to 0.9%, a clinical benefit that was consistently observed during both Omicron BA.2 and BA.2.12.1 and BA.4 and BA.5 predominant periods. All-cause mortality following treatment with nirmatrelvir–ritonavir was 5 times lower in the treatment group (2 deaths vs.15 in the non-treatment group or 0.03% vs. 0.16% in the non-treatment cohort). Fewer emergency department visits following treatment suggests that clinically significant rebound requiring urgent medical care was not observed more frequently among users of oral antivirals.
An accompanying editorial from Hong Kong stresses the ongoing need for real-world studies for two major reasons: 1. Recombinant variants of Omicron – especially XBB.1.5 and BQ.1.1 – continue to emerge posing an imminent threat to public health, due their even greater immune evasion capabilities than BA.5. 2. Such studies remain relevant when assessing cost-effectiveness for different therapeutic strategies and their prioritization among various patient populations, as the number needed to treat to prevent one case of severe COVID-19 might also increase as population immunity grows.
SAB Comment: Using the author’s results, the number of patients necessary to treat to prevent one hospitalization is 200, which is consistent with an absolute risk reduction of 0.5%.
March 10, 2023:
- COVID-19 Incidence and Mortality Among Unvaccinated and Vaccinated Persons Aged ≥12 Years by Receipt of Bivalent Booster Doses and Time Since Vaccination – 24 U.S. Jurisdictions, October 3, 2021-December 24, 2022. 2/9/2023. Johnson AG. MMWR Morb Mortal Wkly Rep.
During the late BA.4/BA.5 period, unvaccinated persons had higher COVID-19 mortality and infection rates than persons receiving bivalent doses (mortality RR = 14.1 and infection RR = 2.8) and to a lesser extent persons vaccinated with only monovalent doses (mortality RR = 5.4 and infection RR = 2.5). Among older adults, mortality rates among unvaccinated persons were significantly higher than among those who had received a bivalent booster (65–79 years; RR = 23.7 and ≥80 years; 10.3) or a monovalent booster (65–79 years; 8.3 and ≥80 years; 4.2). The authors of this CDC MMWR conclude that, “For the best protection against severe COVID-19, all persons should stay up to date with recommended COVID-19 vaccination, including receipt of a bivalent booster by eligible persons.”  Determinants of Professional Fulfillment and Burnout Among Intensivists: A National Survey by the Society of Critical Care Anesthesiologists in 2022. 2/15/23. Siddiqui S. Anesth Analg.
Determinants of Professional Fulfillment and Burnout Among Intensivists: A National Survey by the Society of Critical Care Anesthesiologists in 2022. 2/15/23. Siddiqui S. Anesth Analg.
This is a cross-sectional survey of Society of Critical Care Anesthesiologists members in ICU practices in the US using the Stanford Personal Fulfillment Index. Response rate was 29%, 175 of 606. Several factors were associated with higher levels of personal fulfillment (likely to be an inverse of “burnout”) including age >45 years, ≤15 weeks per year of full time ICU coverage, and nighttime on-call supervision from home rather than in hospital. Adequate staffing allowed the coverage from home and was deemed better for clinician well-being. A favorable public perception of intensivists may have contributed positively and eventually provided additional emotional reward. The survey was comprehensive and assessed personal fulfillment, work exhaustion and interpersonal disengagement. This study sheds light on important underlying causes of healthcare worker loss and reinforces the need for further study.- Early Treatment with Pegylated Interferon Lambda for Covid-19. 2/13/23. Reis G. N Engl J Med.
In this randomized, controlled, adaptive platform, Phase III, outcome based trial, vaccinated adult outpatients who presented with COVID-19 acute respiratory symptoms within 7 days of onset were treated either with pegylated interferon lambda (n=931, single subcutaneous injection, 180 μg) or placebo (n=1,018). The primary outcome of a COVID-19-related emergency department visit or hospitalization within 28 days of randomization occurred in 2.7% following the drug vs. 5.6% following placebo (OR of 0.49). These authors concluded that early treatment with Interferon conveyed a > 50% reduction risk of ER visit or hospitalization, irrespective of vaccine status (0, 1, 2, or 3 shots) and was also effective against multiple variants (Alpha, Delta and Omicron). The authors noted an additional benefit in lowering the viral load. The benefit differed amongst SARS-CoV-2 variants with Omicron > Delta > Alpha. - Infection-induced immunity is associated with protection against SARS-CoV-2 infection and decreased infectivity. 2/12/23. Frutos AM. Clin Infect Dis.
Investigators present data from an ongoing household cohort study between March 2020-November 2022 in Nicaragua to determine transmission after one household member tests positive for SARS-CoV-2. There were 2,399 active participants in the cohort with 87 new/re-enrollees, 394 withdrawn, and 27 deaths. Within this subsidy of SARS-CoV-2 transmission, a total of 228 households (51.9% of all cohort houses) were activated (some multiple times) with 349 total activations. Of activated household contacts, 81.5% consented to intensive monitoring. Transmission occurred in 70% of households, with 41% of household contacts becoming infected. The secondary attack risk ranged from 8% to 14% depending on the time period. Symptomatic infected individuals were more infectious (RR 21.2) and participants with a prior infection were half as likely to be infected compared to naïve individuals (RR 0.52). While young children were less infectious, neither prior infection nor asymptomatic presentation reduced their infectivity (as was seen for adults). Authors comment that as SARS-CoV-2 becomes endemic, children may become more important in transmission dynamics. - Past SARS-CoV-2 infection protection against re-infection: a systematic review and meta-analysis. 2/16/23. Stein M. Lancet.
This meta-analysis of 65 studies (up to September 31, 2022) provides a comprehensive review of the effectiveness of past infection on outcomes (infection, symptomatic disease and severe disease), variant, and time since previous infection. The analysis shows high levels of protection against reinfection from all pre-Omicron variants (82%), but significantly reduced protection against reinfection from Omicron BA.1 (42%). Levels of protection against severe disease remained high for all variants (pooled protection of 78%), including Omicron BA.1. When analyzed as a function of time since previous infection, pre-Omicron infections afforded initially high protection (85%) which waned to 79% at 40 weeks. By contrast, protection from the Omicron BA.1 variant declined more rapidly, decreasing to 36% at 40 weeks. Although protection from reinfection by all variants wanes over time, the level of protection afforded by previous infection is at least as high, if not higher, than that provided by two-dose vaccination using mRNA vaccines.
SAB Comment: The number of studies on vaccine efficacy far exceeds the number of studies on the protection against COVID-19 by previous infection, yet protection afforded by previous infection is likely to be at least as important as vaccination status, and could be factored into public health approaches to COVID-19. This study provides the information needed to address protection from previous infections, at least through Omicron BA.1. - Risk of venous thromboembolism in non-respiratory and respiratory presentations of COVID-19 in critically ill patients. 2/14/23. Roubinian NH. Chest.
This research letter reports a retrospective cohort study of adult Kaiser Permanente patients who were PCR tested for SARS-CoV-2 before admission to 21 ICUs between December 1, 2020 and April 30, 2022. Authors assessed the incidence of venous thromboembolism (VTE) within 90 days of hospital admission, comparing those who presented with respiratory versus nonrespiratory diagnoses. Of these 11,143 cases, 5,440 (49%) were admitted with respiratory diagnoses. 2,983 (27%) had COVID-19, of whom 2,428 (81%) were admitted with respiratory diagnoses. ICU patients admitted with respiratory diagnoses has a higher 90-day incidence of VTE compared with those with nonrespiratory diagnoses (13.4% vs. 7.4%). SARS-CoV-2 infection was not associated with an increased risk of VTE among patients with nonrespiratory diagnoses, regardless of vaccination status. In contrast, the relative risk (RR) of VTE was increased in those admitted to ICU with respiratory diagnoses, including unvaccinated COVID-19 patients (RR 2.9), vaccinated COVID-19 patients (RR 2.0) and those without COVID-19 (RR 1.3). The increased risk of VTE in unvaccinated and vaccinated COVID-19 patients persisted during the period of Omicron variant predominance.
SAB Comment: This dataset helps further characterize the risk of VTE for patients with COVID-19 based upon symptoms at presentation. This study may act as a basis for further studies to better target prophylaxis for VTE in critically ill COVID-19 patients.
February 24, 2023:
- Adherence to Healthy Lifestyle Prior to Infection and Risk of Post-COVID-19 Condition. 2/6/23. Wang S. JAMA Intern Med.
The role of a healthy lifestyle before infection in reducing post-COVID condition (PCC) was the focus of this substudy within the Nurses’ Health Study II, a prospective cohort which enrolled participants in 1989. The substudy enrolled 32,249 nurses for whom lifestyle data from 2017 was available. They completed monthly and quarterly surveys beginning in April 2020, reporting testing positive for SARS-CoV-2 (1,981), and the presence of symptoms for at least 4 weeks (871 or 44%). The 2017 lifestyle parameters (BMI, smoking, alcohol consumption, diet, physical activity and sleep) were individually and collectively compared between those with and without PCC. As the number of healthy lifestyle factors increased, the likelihood of PCC decreased; those with 5-6 healthy parameters had a 49% lower risk of PCC. The most significant factors were BMI (risk ratio [RR] 0.85) and sleep (RR 0.83).
SAB Comment: This report contains a great deal of data, and includes 10 sensitivity analyses, as well as population attributable risk (PAR) information. The authors posit that the anti-inflammatory effect of a healthy lifestyle mitigates PCC. It must be kept in mind that the population was 97% white and limited to women aged 55-75, who were willing to share their health information for more than 30 years. - Beyond acute COVID-19: A review of long-term cardiovascular outcomes. 2/8/2023. Parhizgar P. Can J Cardiol.
This is a review of cardiac abnormalities persisting beyond one month after acute COVID-19. They include myocarditis, asymptomatic troponin elevation, myocardial edema, pericarditis, inappropriate sinus tachycardia and bradycardia, decreased heart rate variability, atrial and ventricular dysrhythmias, postural orthostatic tachycardia syndrome, prolonged QT interval, myocardial ischemia, myocardial fibrosis, R and L ventricular dysfunction, heart failure, new onset hypertension, and pulmonary hypertension. Patients who had mild COVID are not exempt. Findings appear to improve over time in most but not all. Potential mechanisms are discussed. - Early Estimates of Bivalent mRNA Booster Dose Vaccine Effectiveness in Preventing Symptomatic SARS-CoV-2 Infection Attributable to Omicron BA.5- and XBB/XBB.1.5-Related Sublineages Among Immunocompetent Adults – Increasing Community Access to Testing Program, United States, December 2022-January 2023. 2/2/23. Link-Gelles R. MMWR Morb Mortal Wkly Rep.
This is a CDC report on the bivalent mRNA vaccine effectiveness (VE) against symptomatic infection by recent SARS-CoV-2 variants BA.5 and XBB/XBB1.5. Using data from the Increasing Community Access to Testing Program during December 1, 2022 to January 13, 2023, the authors used a test negative control format to evaluate 29,175 immunocompetent adult patients with COVID-19-like symptoms who had received two or more monovalent mRNA vaccine/booster doses. In persons 18-49 years of age who had additionally received a bivalent mRNA booster 2 to 3 months earlier compared with no bivalent booster, VE was 52% against symptomatic BA.5 infection and 48% against symptomatic XBB/XBB1.5 infection. Other age groups also had modest but real VE with a bivalent booster.
SAB Comment: As featured in two articles in Newsletter Issue 159 (“Early Estimates of Bivalent mRNA Vaccine Effectiveness in Preventing COVID-19-Associated Hospitalization Among Immunocompetent Adults Aged ≥65 Years – IVY Network, 18 States, September 8-November 30, 2022” and “Early Estimates of Bivalent mRNA Vaccine Effectiveness in Preventing COVID-19-Associated Emergency Department or Urgent Care Encounters and Hospitalizations Among Immunocompetent Adults – VISION Network, Nine States, September-November 2022“) bivalent boosters reduced the incidence of hospitalizations and urgent care or emergency department evaluations. This is the first evaluation of VE of the bivalent booster against symptomatic infections with the most recent BA.5 and XBB/XBB1.5 lineages and suggests that all persons should stay up to date with recommended COVID-19 vaccines. - Physical interventions to interrupt or reduce the spread of respiratory viruses. 1/30/23. Jefferson T. Cochrane Reviews.
This Cochrane Review metanalysis has generated significant press controversy despite the clearly stated concerns of its authors that more and better studies are essential to gaining an adequate understanding of the effects of these interventions. This review is an update of one last published in 2020 and only 2 of the 9 studies used to develop the updated first and primary conclusion concerned COVID-19 infection. That conclusion was that wearing masks in the community probably makes little or no difference to the outcome of influenza‐like illness or COVID‐19 like illness compared to not wearing masks (risk ratio (RR) 0.95, 9 trials, 276,917 participants); or in the outcome of laboratory‐confirmed influenza/SARS‐CoV‐2 (RR 1.01, 6 trials, 13,919 participants). However, it’s worth noting that both COVID-19 studies used were designed to test a mask encouraging strategy, not mask wearing, and both showed a small decrease in infection rate in the intervention group which was statistically significant in the larger of the two studies (but not the smaller).
Two additional conclusions are presented: 1) The use of a N95/P2 respirators compared to medical/surgical masks probably makes little or no difference for the objective outcome of laboratory‐confirmed influenza infection (RR 1.10, 5 trials, 8407 participants), and 2) Pooled data showed that hand hygiene may be beneficial with an 11% relative reduction of respiratory illness (RR 0.89, low‐certainty evidence with high heterogeneity).
The authors state that, “The high risk of bias in the trials, variation in outcome measurement, and relatively low adherence with the interventions during the studies hampers drawing firm conclusions.”
SAB Comment: We’re impressed, as are the authors, that there are so few randomized controlled trials published to date of the effectiveness of mask wearing during the COVID-19 epidemic but wonder, given the lethality of the virus, whether researchers have been hesitant to define a control group. - Reports of Guillain-Barré Syndrome After COVID-19 Vaccination in the United States. 2/1/23. Abara WE. JAMA Netw Open.
Relying on the Vaccine Adverse Event Reporting System (VAERS), this CDC and FDA-authored retrospective cohort study analyzes the occurrence of Guillain-Barré Syndrome (GBS) within 21-42 days following 3 different COVID-19 vaccines administered between December 2020 and January 2022. Among 488 million vaccinations, there were 295 verified cases of GBS and a 9-12 times higher incidence following vaccination with Ad26.COV2.S (Janssen) compared to BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna). While contributing cause and associate risk factors remain unclear, the authors identify a number of potential mechanisms related to the immune response elicited by the vaccine on a molecular level. The Advisory Committee on Immunization Practices recommends the mRNA COVID-19 vaccine rather than Ad26.COV2.S when both are available but bases this recommendation primarily on the increased risk of developing thrombosis with thrombocytopenia syndrome in addition to the risk of developing GBS. - Vaccination status and long COVID symptoms in patients discharged from hospital. 2/11/2023. Nascimento TCDC. Sci Rep.
From May 2021 to February 2022, 412 consecutive SARS-CoV-2 positive patients hospitalized in 2 Brazilian locations were enrolled in a study reporting symptoms at 90 days post infection via telephone interview. The patients who had complete vaccination were compared to those with partial or no vaccination. Although younger and with less comorbidities, 31% of the unvaccinated group were admitted to the ICU, as opposed to 20% of the vaccinated. At 90 days the odds ratio of having more than 1 symptom was 2.4 for females, 1.8 for unvaccinated, and 1.7 for ICU admission. The authors conclude vaccination mitigates the probability of long COVID.
February 6, 2023:
- Cardiologic Manifestations in Omicron-Type Versus Wild-Type COVID-19: A Systematic Echocardiographic Study. 1/25/2023. Ghantous E. J Am Heart Assoc.
Study designed to compare echocardiographic findings in patients hospitalized with COVID-19 between early Wild-Type disease (03/21-09/16/20) and the later Omicron variant 01/03-05/22. 162 consecutive patients hospitalized with Omicron COVID-19 were compared with propensity-matched patients (148 total pairs) with the wild-type variant (COVID-19 Wuhan_hu_1) previously studied used as control. Both patient cohorts evaluated within 48 hours by trained echocardiographers following defined and comprehensive protocol followed by off line speckle tracking evaluation and supplemented by routine biochemical and radiographic investigations. Patients with the wild-type variant had larger RV, poorer RV function, and higher pulmonary vascular resistance compared with patients in the Omicron-variant matched cohort irrespective of grade of disease. In the matched cohort, in hospital death, requirement for invasive ventilation, and combined events was higher in the wild type disease. Patients with acute wild-type infection and no cardiac disease or cardiovascular risk factors had echocardiographic LV and RV parameters, suggesting that acute wild-type infection causes acute elevation of RV afterload, resulting in lower left-filling pressure and stroke volume. However, in a similar group of patients with acute Omicron-type infection, these changes were not recorded. In the majority of patients with abnormal LV systolic function, or elevated filling, and a previous echocardiographic exam, similar abnormalities were recorded in the exam before the current admission, suggesting that in most patients with Omicron, LV dysfunction is related to background cardiac disease and that acute infection does not cause significant additive LV injury. - Long covid outcomes at one year after mild SARS-CoV-2 infection: nationwide cohort study. 1/11/2023. Mizrahi B. BMJ.
This nationwide Israeli retrospective cohort review of electronic health records comparing approximately 300,000 matched PCR negative or positive patients with mild disease, addresses long COVID outcomes. Patients were enrolled from March 2020 to October 2021, and 70 symptoms associated with long COVID were identified via ICD-10 codes, and analyzed at 1-6 months and 6-12 months post testing. The COVID variant was determined by dominance at the time of testing. The median age of the population was 25. Symptoms more frequent in PCR positive persons at 1-6 months were respiratory disorders, hair loss, chest pain, myalgia and cough. Symptoms occurring significantly more often in PCR positive persons at 6-12 months were anosmia, dysgeusia, concentration and memory impairment, dyspnea, weakness, palpitations, streptococcal tonsillitis, and dizziness. The risk of receiving a prescription for a pulmonary diagnosis was independent of PCR test result. There was no difference in symptom incidence between COVID variants. Comparison of 14,000 vaccinated versus unvaccinated PCR positive patients revealed less dyspnea in the vaccinated population.  Long COVID: major findings, mechanisms and recommendations. 1/13/23. Davis HE. Nat Rev Microbiol.
Long COVID: major findings, mechanisms and recommendations. 1/13/23. Davis HE. Nat Rev Microbiol.
This literature review summarizes the current knowledge about most aspects of long COVID. Starting with epidemiology (an estimated incidence of 10-30% of nonhospitalized cases, 50-70% of hospitalized cases and 10-12% of vaccinated cases), the authors move through the known and postulated etiologies of long COVID, including immunologic dysregulation, reactivation of SARS-CoV-2 and other associated viruses, clotting and endothelial abnormalities, dysfunctional neurological signaling and other etiologies. Long COVID of the commonly affected organ systems is reviewed, as well as the diagnostic tools and treatments currently in use. Some of the “miscues” about long COVID are briefly mentioned. The impact of vaccination and re-infection on established long-COVID patients is discussed. Particular attention is paid to myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and dysautonomia, especially postural orthostatic tachycardia syndrome (POTS). The authors clearly state that much more research is needed to understand and treat long COVID.
SAB Comment: This exhaustive review packs a huge amount of information into just a few pages, with useful tables and graphs. Some of the authors are members of the Patient-Led Research Collaborative, a team of long-COVID patients with a wide range of research, policy, design and medical backgrounds.- VV116 versus Nirmatrelvir-Ritonavir for Oral Treatment of Covid-19. 12/28/22. Cao Z. N Engl J Med.
VV116 is an orally effective antiviral remdesivir analogue developed in China and undergoing multicenter, observer blinded, randomized controlled trial on symptomatic patients at high risk for progression to severe COVID-19. To compare duration of symptoms and time to clinical recovery, SARS-CoV-2 infected patients with a median age of 53 and mostly vaccinated, were recruited from 7 Shanghai hospitals during the month of April 2022 and assigned to receive either the study drug (n=384) or nirmatrelvir-rotinavir (Paxlovid) (n=387) orally twice a day. The result showed that among symptomatic adults hospitalized with mild to-moderate COVID-19 with risk factors including age older than 50 years, cardiac disease and obesity, a 5-day course of oral treatment with VV116 was noninferior to nirmatrelvir–ritonavir in shortening the time to sustained clinical recovery (HR 1.17, [CI 1.01 to 1.35]). Among the limitations listed in this industry-funded study is the inability to draw conclusions about the efficacy of VV116 for the prevention of progression to severe or critical COVID-19 or death, because no events occurred in either group.
SAB Comment: This is one of several recent studies exploring oral antiviral alternatives to Paxlovid and remdesivir. Worldwide shortages, high costs and the need for parenteral administration of remdesivir are of concern for currently approved antivirals, as well as a high percentage of contraindications due to impaired renal and hepatic function and drug-drug interactions among patients who would otherwise be prime candidates for these treatments based on their risk category.
January 23, 2023:
- Diaphragm Muscle Weakness Might Explain Exertional Dyspnea Fifteen Months After Hospitalization for COVID-19. 1/3/23. Regmi B. Am J Respir Crit Care Med.
The etiology of long COVID/Post-COVID Condition (PCC) remains an enigma. Recent studies consider multiple causes which remain under investigation. Additional questions remain whether invasive ventilation increases the incidence or severity of symptoms. Many COVID-19 survivors report dyspnea that cannot be explained by routine clinical diagnostic measures, including pulmonary function tests and cardiac evaluation. This study investigated 50 patients previously hospitalized with COVID-19 (14 female, age 58±12 years), half were treated with mechanical ventilation and the remaining outpatients using pulmonary function testing, 6-minute walk test, echocardiography, twitch transdiaphragmatic pressure following cervical magnetic stimulation of the phrenic nerve roots, and diaphragm ultrasound. Diaphragm function data were compared with values from a healthy control group. Testing evaluated both voluntary and involuntary (magnetic phrenic nerve stimulation) measurements of inspiratory and expiratory power at FRC. Dyspnea was equally experienced between survivors who required invasive ventilation (including ECMO) and those managed without. Diaphragmatic inspiratory strength reflected in voluntary and magnetically induced twitch pressures generated at functional residual capacity (FRC) and diaphragmatic thickening noted on ultrasound were reduced. Expiratory values similarly measured showed minimal disruption of expiration. The study demonstrated diaphragm muscle weakness underlying otherwise unexplained exertional dyspnea in patients previously hospitalized for COVID-19 and suggested specific interventions, such as inspiratory muscle training, could be an effective approach to mitigate COVID-19 exertional dyspnea. - Imprinted SARS-CoV-2 humoral immunity induces convergent Omicron RBD evolution. 12/19/2022. Cao Y. Nature.
Immune imprinting occurs when exposure to an original viral strain prevents our bodies from producing sufficient neutralizing antibodies (NAb) against diverse new strains of that virus.
Unprecedented numbers of Omicron BA.5 variants are emerging (including BQ.1.1.10, and XBB) that for unclear reasons display mutations that converge at same antibody-evasive hotspots on the receptor-binding domain (RBD). These investigators demonstrate that vaccination with BA.2 and BA.5 mainly recalls the same cross-reactive memory B cells elicited previously by wildtype-based vaccine, but rarely produce novel BA.2/BA.5 specific B cells, and therefore, no new divergent antibodies specific for the mutant hotspots. However, the NAbs still maintain sufficient ACE2 binding capability and will fight the novel-mutant infections. Immune imprinting also reduced the diversity of the NAb binding sites and increased proportions of non-neutralizing antibody clones. Reduced diversity focused humoral immune pressure and promoted convergent mutational evolution in the untargeted RBD hotspots.
SAB Comment: These results suggest that our current herd immunity whether produced by natural infection and/or by inactivated vaccines or mRNA vaccines, may impede our ability to fight emerging Omicron variants. Immune imprinting (a.k.a., “original sin”) will inhibit our capacity to synthesize novel antibodies to recognize these new convergent mutations in Omicron variants. Recent clinical trials bear out this prediction. The bivalent boosters may be no better than the monovalents in eliciting novel variant-fighting antibodies. - Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial. 12/25/2022. Butler CC. Lancet.
Reviews prior to this study demonstrated molnupiravir reduction in hospitalization following early treatment of PCR positive patients. The largest randomized clinical trial was the placebo-controlled, phase 3 MOVe-OUT trial of 1,433 unvaccinated outpatients with COVID-19, molnupiravir was associated with a relative reduction of roughly 30% in the primary outcome — hospitalisations and deaths — up to 29 days after randomization. Subsequent reports showed less efficacy. This study PANORAMIC was a UK-based, national, multicentre, open-label, multigroup, prospective, platform adaptive randomised controlled trial enrolling ~25,000 patients assigned randomly in two equal arms from 12/08/2021 to 04/27/2022 to receive either usual care only or usual care + 800 mg molnupiravir orally twice daily for 5 days witin 5 days of PCR Confirmed COVID 19. Eligible people were in the community (ie, not in hospital), aged 50 years or older (or 18 years or older with relevant comorbidities), had COVID-19 symptoms that had started within the previous 5 days, and had had a positive PCR or rapid antigen SARS-CoV-2 test within the past 7 days. The primary outcome was all-cause, non-elective hospital admission or death within 28 days of randomisation. Secondary outcomes included time to self-reported recovery, time to sustained alleviation of symptoms, time to initial reduction of symptom severity, contact with health or social services, hospital assessment without admission, oxygen administration, new household COVID-19 infections, and safety outcomes. Virology studies were also performed. This trial of vaccinated adults at increased risk of an adverse outcome and unwell with confirmed SARS-CoV-2 infection showed that early treatment with molnupiravir did not reduce already low hospital admission or deaths. Their findings suggest that, in a highly vaccinated population at high risk (but not the highest risk) of complications from COVID-19, the avoidance of hospitalization and death is primarily achieved via extensive vaccination. The benefits of molnupiravir in terms of faster time to recovery, reduced contact with general practitioner services, and reduced viral load need to be considered in the context of the prevailing disease, burden on health-care services, drug-acquisition cost, social circumstances, cost-effectiveness, and opportunity costs. - Optimized ACE2 decoys neutralize antibody-resistant SARS-CoV-2 variants through functional receptor mimicry and treat infection in vivo. 12/7/22. Torchia JA. Science Advances.
These investigators report the development of recombinant soluble forms of the cell surface ACE-2 protein to act as a therapeutic decoy receptor. The decoys show no loss of potency with recent variants. The decoy receptors engage the viral receptor-binding domain (RBD) and are thought to outcompete cell-surface ACE2 for viral binding. The authors state the decoy is in theory, identical to the cell-surface receptor. Therefore, viral mutations which would weaken binding to the decoy would weaken binding to the cell surface receptor and reduce infectivity. The optimized ACE2 decoy triggers irreversible structural changes in the critical viral RBD S-protein. These studies show how ACE2 decoys function and support therapeutic development for emerging ACE-2-dependent coronaviruses. - Prevalence of Contraindications to Nirmatrelvir-Ritonavir Among Hospitalized Patients With COVID-19 at Risk for Progression to Severe Disease. 11/15/22. Hoertel N. JAMA Netw Open.
Nirmatrelvir-ritonavir (Paxlovid) reduces COVID-related morbidity and mortality. Hospitalized patients may have contraindications to its use. Ritonavir may elevate drug concentrations if dependent on hepatic cytochrome P-450-3A (CYP3A) metabolism. Coadministration with CYP3A inducers can significantly reduce nirmatrelvir concentrations with loss of drug response. Severe kidney and liver disease patients were excluded from clinical trials. In 36 Parisian University Hospitals, the authors studied records from January 24, 2020 to November 30, 2021, a period before patients received nirmatrelvir-ritonavir. Of 62,500 PCR-proven patients; 9,136 (14.6%) had a medical contraindication (men 18%, women 11%; older (>65)- 27%; co-morbidities-37%). Among 4,861 patients who died, 50.7% had a contraindication. The authors conclude that published nirmatrelvir-ritonavir hospital studies may overestimate treatment efficacy by avoiding patients with contraindications. A large unmet need remains for effective COVID treatments.
January 9, 2023:
- SAB Comment for the following three studies summarized below: The two well-done clinical studies provide much anticipated bivalent vaccine effectiveness data as Omicron variants continue to evolve and many infections are uncounted. Despite lab data that suggest viral neutralization after the bivalent vaccine is poor (lab study below titled “Alarming Antibody Evasion…), these clinical studies document real but moderate protection against visits to urgent care or emergency departments and hospitalization. This protection seems to be most prominent in older adults and the time since the previous monovalent vaccine dose was of greater significance. These studies do not address the durability of bivalent booster effectiveness. This information suggests that all eligible persons should stay up to date with recommended COVID-19 vaccinations despite indications that we may be headed for a period of increased breakthrough infections.
- Early Estimates of Bivalent mRNA Vaccine Effectiveness in Preventing COVID-19-Associated Emergency Department or Urgent Care Encounters and Hospitalizations Among Immunocompetent Adults – VISION Network, Nine States, September-November 2022. 12/29/22. Tenforde MW. MMWR Morb Mortal Wkly Rep.
This is a bivalent vaccine effectiveness (VE) study from the VISION Network (nine US states) during September 13 to November 18, 2022, specifically looking at effectiveness against Emergency Department (ED) and Urgent Care (UC) encounters as well as hospitalizations. Among 78,303 ED/UC encounters in immunocompetent adults (over 18 years old), VE of the bivalent vaccine was 56% compared to no vaccination, 31% compared with receipt of last monovalent dose 2-4 months earlier, and 50% compared with receipt of last monovalent dose 11 months or more earlier. Among 15,527 hospitalizations of immunocompetent adults, the booster VE was similar to that in ED/UC patients. - Early Estimates of Bivalent mRNA Vaccine Effectiveness in Preventing COVID-19-Associated Hospitalization Among Immunocompetent Adults Aged ≥65 Years – IVY Network, 18 States, September 8-November 30, 2022. 12/29/22. Surie D. MMWR Morb Mortal Wkly Rep.
These IVY Network investigators from 18 US states sought to define vaccine effectiveness of the bivalent COVID-19 vaccine against hospitalization in elderly patients. Between September 8 and November 30, 2022, 798 patients hospitalized with a COVID-like illness were enrolled in this test-negative analysis. Among immunocompetent adults aged ≥65 years, a bivalent booster dose received after ≥2 monovalent mRNA doses provided good protection (73%) against COVID-19-associated hospitalization during this period of Omicron BA.5 or BQ.1/BQ.1.1 predominance. Compared to unvaccinated patients, the bivalent vaccine effectiveness was 84%. Substantial additional protection from a bivalent booster dose was observed when compared with remote monovalent-only mRNA vaccination. - Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants. 12/29/22. Wang Q. Cell.
This is a detailed in-vitro study of antibody resistance and viral receptor binding affinity of SARS-CoV-2 Omicron BQ.1, BQ.1.1, XBB, and XBB.1 subvariants, that are now prevalent and rapidly expanding globally. Sera from 5 cohorts (n=14-21 each) with the following histories were tested: 1) 3 doses of an original COVID-19 mRNA vaccine, 2) 4 doses of an original COVID-19 mRNA vaccine, 3) a fourth vaccination with a bivalent COVID-19 mRNA vaccine, 4) breakthrough BA.2 infection post-vaccination, and 5) breakthrough BA.4 or BA.5 infection. Results showed BQ.1.1 is approximately 6-fold more resistant to serum neutralization than its predecessor BA.5. XBB.1 is ∼63-fold more resistant to serum neutralization than its predecessor, or ∼49-fold more resistant than BA.4/5. SARS-CoV-2 breakthrough infection induced somewhat better antibody responses than vaccination. In addition, the clinically authorized monoclonal antibodies bebtelovimab and Evusheld could not neutralize BQ or XBB variants, leaving no effective preventative treatments for immunocompromised patients. The publication includes illustrative graphs, tables, and structural analysis. Analysis of cellular immunity and clinical studies are called for; however previous in-vitro studies have been predictive of in vivo outcomes.
- Early Estimates of Bivalent mRNA Vaccine Effectiveness in Preventing COVID-19-Associated Emergency Department or Urgent Care Encounters and Hospitalizations Among Immunocompetent Adults – VISION Network, Nine States, September-November 2022. 12/29/22. Tenforde MW. MMWR Morb Mortal Wkly Rep.
- SAB Comment for the following two articles summarized below: The two studies below are among those that assess the real-world effect of Paxlovid during the Omicron era. They show similar reductions in hospitalization rate which are not nearly as large as that seen in the Paxlovid phase 2-3 trial done with unvaccinated patients during the Delta variant surge in 2021 (87.8% reduction of combined death or hospitalization). In these recent studies the rates of hospitalization or mortality are significantly reduced in all patients (Paxlovid treated or untreated) compared to the original phase 2-3 study likely related to a combination of vaccinations, prior infections and decreasing virulence of evolving viral strains. It’s interesting that as a consequence, both of the studies below show that the number of patients needed to treat to achieve a single improved outcome (NNT) in hospitalization or combined hospitalization/mortality exceeds 200 patients.
 Paxlovid Associated with Decreased Hospitalization Rate Among Adults with COVID-19 — United States, April–September 2022. 12/2/22. Shah M. MMWR Morb Mortal Wkly Rep.
Paxlovid Associated with Decreased Hospitalization Rate Among Adults with COVID-19 — United States, April–September 2022. 12/2/22. Shah M. MMWR Morb Mortal Wkly Rep.
This study reviewed electronic medical records to show that of 699,848 adults aged ≥18 years who were eligible for Paxlovid during April-August 2022, 28.4% received a Paxlovid prescription within 5 days of COVID-19 diagnosis (of whom 68.8% previously had received two or more doses of mRNA vaccine). Being prescribed Paxlovid was associated with a lower hospitalization rate among the overall study population (adjusted hazard ratio [aHR] = 0.49). The reduction was similar among those who had received ≥3 mRNA COVID-19 vaccines (aHR = 0.50) and across all age groups. The proportion of persons with in-hospital death was also lower among persons who received Paxlovid (0.01%) than among those who did not (0.04%). The authors state that Paxlovid should be prescribed to eligible adults to reduce the risk of COVID-19-associated hospitalization.
SAB Comment: This study shows a significant real-world effect of Paxlovid during the Omicron era but with a reduction in hospitalization rate that is not nearly as large as that seen in the Paxlovid phase 2-3 trial done with unvaccinated patients during the Delta variant surge in 2021 (87.8% reduction of combined death or hospitalization).- Nirmatrelvir Plus Ritonavir for Early COVID-19 in a Large U.S. Health System : A Population-Based Cohort Study. 12/12/22. Dryden-Peterson S. Ann Intern Med.
This study was designed to assess the real-world efficacy of nirmatrelvir plus ritonavir (Paxlovid) for early outpatient COVID-19 during the first 6 months of 2022 in an era of prevalent SARS-CoV-2 immunity and immune-evasive Omicron subvariants. It attempts to emulate a clinical trial using observational data from 117,000 patients diagnosed with COVID-19 to determine the drug combination’s efficacy in preventing hospitalization within 2 weeks of diagnosis and death of any causes within 28 days. Of 44,000 outpatients receiving Paxlovid, 69 (0.55%) were hospitalized compared to 310 (.97%) among the 32,000 patients who were not, resulting in an adjusted risk ratio for Paxlovid of 0.56 (CI, 0.42-0.75), confirming the drug combination’s effectiveness among vaccinated and unvaccinated patients aged 50 and older.
An accompanying editorial compliments the author’s attempt to minimize bias in a retrospective data evaluation, lends perspective to the value of recent trials and reviews current literature.
SAB Comment: This is a high-profile retrospective real-world study corroborating Paxlovid’s effectiveness.
- Association of Time to Surgery After COVID-19 Infection With Risk of Postoperative Cardiovascular Morbidity. 12/14/2022. Bryant JM. JAMA Netw Open.
This retrospective study from Vanderbilt was conducted among 3997 adult patients with a history of COVID-19, who were undergoing surgery from January 2020 to December 2021. The primary outcome was the composite occurrence of DVT, PE, CVA , MI, acute kidney injury, and death within 30 days after surgery.
The median time from COVID-19 diagnosis to surgery was 98 days. Major postoperative cardiovascular events were identified in 12.1%. Increased time from COVID-19 diagnosis to surgery was associated with a decreased rate of the composite outcome (adjusted odds ratio, 0.99 [per 10 days]; 95% CI, 0.98-1.00; P_=_.006). This study suggests that increased time from COVID-19 diagnosis to surgery was associated with decreased odds of experiencing major postoperative cardiovascular morbidity.
SAB Comment: The data is from a prior COVID-19 variant and may not be relevant to current practice. The authors were not able to suggest a time when the procedures would be safer. The specifics of the procedures are not detailed limiting the applicability of these findings. The rate of decrease in the composite endpoint over time was noted to be quite slow. These data still suggest the individualized assessment for each patient with respect to the type of the procedure, anesthesia and their co-morbidities. - Early Estimates of Bivalent mRNA Vaccine Effectiveness in Preventing COVID-19-Associated Emergency Department or Urgent Care Encounters and Hospitalizations Among Immunocompetent Adults – VISION Network, Nine States, September-November 2022. 12/29/2022. Tenforde MW. MMWR Morb Mortal Wkly Rep.
This is a bivalent vaccine effectiveness (VE) study from the VISION Network (nine US states) during September 13 to November 18, 2022, specifically looking at effectiveness against Emergency Department (ED) and Urgent Care (UC) encounters as well as hospitalizations. Among 78,303 ED/UC encounters in immunocompetent adults (over 18yo), VE of the bivalent vaccine was 56% compared to no vaccination, 31% compared with receipt of last monovalent dose 2-4 months earlier, and 50% compared with receipt of last monovalent dose 11 months or more earlier. Among 15,527 hospitalizations of immunocompetent adults, the booster VE was similar to that in ED/UC patients.
- Impact of SARS-CoV-2 variants on inpatient clinical outcome. 12/18/2022. Robinson ML. Clin Infect Dis.
This study, from EHR and databases for 5 Johns Hopkins hospitals between 9/2020 and 5/2022, assesses approximately 4,500 patients hospitalized for COVID-19 grouped by SARS-CoV-2 variant, and previous infection or vaccination. The primary outcome was severe disease (treatment with high flow nasal cannula, non-invasive PPV, or IMV), or death. The variant was determined by whole genome sequencing (22%), or admission when > 95% of cases were a single variant. The probability of severe disease for patients without prior vaccination or infection was 0.28 for ancestral, 0.35 for alpha, 0.36 for delta and 0.28 for omicron and the relative risk of severe disease, compared to ancestral, was 1.3 for delta and 0.94 for omicron. When compared to those without prior infection or vaccination, the hazard ratio of severe disease was 0.4 for patients with prior infection or vaccination. There was no difference in the incidence of severe disease between the delta and omicron variants in this group.
- Two masks can be worse than one: N95 respirator failure caused by an overlying face mask. 12/20/22. Mueller JT. Infection Control & Hospital Epidemiology.
Adding an external procedure mask in front of a filtering face-piece respirator (FFR), such as an N95, has been suggested at times, largely to protect the FFR from soiling and to extend its use. One hundred healthcare workers from Mayo Clinic in Arizona, who successfully passed quantitative fit testing of an N95 respirator (3M 1870 Aura FFR), were immediately re-evaluated to assess the effect of adding a procedure mask (Halyard 47117) in front of their N95. Thirteen out of 100 participants failed the quantitative fit test following the addition of the procedure mask. Authors review potential causes as well as limitations of the study with regard to testing a single combination of face protection devices. Guidance on the use of N95 FFRs should consider the potential risk of increased fit failure when they are worn with an overlying face mask. Further research including other FFR + face-mask combinations is needed.
December 19, 2022:
- Apparent risks of postural orthostatic tachycardia syndrome diagnoses after COVID-19 vaccination and SARS-Cov-2 Infection. 12/12/2022. Kwan AC. Nature Cardiovascular Research.
Postural orthostatic tachycardia syndrome (POTS) has been previously described after COVID-19 infection. Here, the authors describe the relation of POTS to COVID-19 vaccination reviewing medical records from a cohort of 284,592 COVID-19-vaccinated individuals and comparing the odds of a new POTS diagnosis 90_days after vaccine exposure to those 90_days before exposure. A POTS diagnosis was more frequent after vaccination (O.R. 1.52) with an absolute increase of slightly less than 0.1% (0.18% to 0.27%). A similar comparison was made for the occurrence of POTS before and after COVID-19 infection in a group of 12,460 individuals with proven infection. The post-infection risk of a new diagnosis of POTS was significantly higher after infection than after vaccination (O.R. 5.35). POTS occurred much less frequently than myocarditis after vaccination but more frequently than myocarditis after infection. - Incidence of Viral Rebound After Treatment With Nirmatrelvir-Ritonavir and Molnupiravir. 12/6/22. Wong GL. JAMA Netw Open.
To better understand the much-discussed rebound phenomena when COVID-19 patients receive oral antiviral medication (molnupiravir (Lagevrio) or nirmatrelvir-rotinavir (Paxlovid)), 12,629 adult COVID-19 inpatients in Hong Kong were followed with RT-PCR testing during the first three months of 2022. Serial cycle threshold (Ct) values were used to measure rebound, defined as a Ct value that increased to > 40, followed by a decrease to 40 or less. Viral rebound was uncommon, occurring in 0.6% of patients who did not receive an antiviral (n=11,688), 0.8% in molnupiravir patients (n=746), and 1% in nirmatrelvir-rotinavir patients (n=195). Viral rebound was not associated with an increased risk of mortality.
SAB Comment: It’s important to note that this study evaluates viral and not symptom rebound in hospitalized patients. Clinical symptoms were not studied. This study is similar to a research letter previously presented in newsletter 148. Both show a low incidence of viral rebound in treated as well as untreated patients. One study of the mechanism of rebound suggests that a more robust immune response rather than uncontrolled viral replication is the cause of clinical rebound.
December 5, 2022:
 A population-based serological study of post-COVID syndrome prevalence and risk factors in children and adolescents. 11/29/22. Dumont R. Nat Commun.
A population-based serological study of post-COVID syndrome prevalence and risk factors in children and adolescents. 11/29/22. Dumont R. Nat Commun.
This report is from a Swiss population study of 1,034 children, aged 6 months to 17 years, who were tested for anti-SARS-CoV-2 N antibodies December 2021-February 2022. Pediatric vaccinations were not available to participants until early January 2022. Their parents filled in a questionnaire on symptoms compatible with post-COVID syndrome lasting over 12 weeks. Of the 1,034 children tested, 570 (55.1%) were seropositive. Overall, 24% has a prior test confirming SARS-CoV-2 infection, and 19% had had clinical symptoms consistent with COVID-19. Both were far more prevalent in the seropositive cohort, (by a factor of ~8x). The sex- and age-adjusted prevalence of children with persistent symptoms was 9.1% among seropositive children and 5.0% among seronegative children, with an adjusted prevalence difference (ΔaPrev) = 4.1%. Stratifying by age group, only those 12-17 years of age displayed a substantial risk of having post-COVID symptoms (ΔaPrev = 8.3%). Identified risk factors for post-COVID syndrome were older age, lower socioeconomic status and chronic health conditions, especially asthma.
SAB Comment: This study is useful in estimating the population’s prevalence of long COVID in young children (0%) and in adolescents (8.3%) of all those with positive serologies but does not estimate a symptomatic COVID-infected adolescent’s risk of long COVID (which may be significantly higher). Because pediatric vaccinations began in Switzerland in January 2022, the study could not assess the effect of prior vaccination on persistent symptoms, as not enough participants were vaccinated.- Cardiovascular outcomes after tixagevimab and cilgavimab use for pre-exposure prophylaxis against COVID-19: a population-based propensity-matched cohort study. 11/16/2022. Birabaharan M. Clin Infect Dis.
Tixagevimab and cilgavimab (T&C), EvusheldTM, is a human monoclonal antibody treatment currently FDA authorized as COVID prophylaxis for individuals unlikely to respond to vaccination (e.g. immunocompromsed) or unable to receive vaccines. In a post-hoc analysis of a phase 3 trial, T&C was associated with higher rates of cardiovascular events, resulting in a warning to potential recipients. Using a database of 74 million Americans, authors identified 8,627 people who received T&C and 295,347 controls between 12/8/21 and 6/8/22. Authors found no increased risk of MI, dysrhythmia, or heart failure at 30 or 90 days after administration, including in patients with pre-existing cardiovascular disease.
SAB Comment: Authors found hazard ratios for serious cardiovascular events for T&C recipients of 0.84 at 30 days and 0.81 at 90 days, but stopped short of stating that T&C may possibly be protective by preventing COVID. A weakness of the study is that the original post-hoc analysis followed patients up to six months while this study ended 90 days post-T&C administration. - Higher versus Lower Dose Corticosteroids for Severe to Critical COVID-19: A Systematic Review and Dose-Response Meta-analysis. 11/30/2022. Pitre T. Ann Am Thorac Soc.
According to this article, compared to lower-dose corticosteroids (6mg/day*10), higher-dose corticosteroids (12 mg/day*10) probably reduce mortality, and may reduce mechanical ventilation. Clinicians and guideline developers should consider benefits of treatment with higher doses of corticosteroids. The ongoing RECOVERY trial will compare high-dose dexamethasone (20 mg/day*5 followed by 10mg/day*5) with the usual treatment of low-dose dexamethasone (6 mg/day*10) and may provide necessary information to support an appropriately timed and increased dose.
View more information about the RECOVERY trial.  Incidence of Epilepsy and Seizures Over the First 6 Months After a COVID-19 Diagnosis: A Retrospective Cohort Study. 11/17/22. Taquet M. Neurology.
Incidence of Epilepsy and Seizures Over the First 6 Months After a COVID-19 Diagnosis: A Retrospective Cohort Study. 11/17/22. Taquet M. Neurology.
To find the incidence of new-onset seizures and epilepsy after COVID-19 infection, these authors used data from a network of linked electronic health records that included over 81 million patients, mostly in the USA. Between January 2020 and May 2021, 152,754 patients with COVID-19 were compared to a matched cohort of 152,754 patients with influenza. The incidence of seizures within six months of COVID-19 was 0.81% (hazard ratio (HR) compared to influenza 1.55) and the incidence of epilepsy was 0.3% (HR compared to influenza 1.87). The incidence of epilepsy after COVID-19 compared to influenza was greater in people who had not been hospitalized and in individuals younger than 16 years old.
SAB Comment: An associated editorial discussed the clinical implications of these findings. Though the incidence of seizures or epilepsy is low, the large number of COVID-19 cases over the pandemic and the finding that milder cases are more at risk means that there may be many additional seizure and epilepsy patients presenting to providers after COVID-19. The etiology of the seizures and the long-term natural history for these patients is presently unknown.- Omicron sublineage BQ.1.1 resistance to monoclonal antibodies. 11/21/22. Arora P. Lancet Infect Dis.
This correspondence was submitted by a group of scientists working in a German primate center. They demonstrated on color charts how monoclonal antibodies (mAbs) suffer from increasing resistance and lack of effectiveness against several omicron sublineages. In particular, BQ.1.1, which is currently spreading in Europe and the US, is completely resistant to all mAbs in the authors’ model which consists of pseudovirus particles exposed to subclinical serum levels of a series of 16 mAbs, administered individually or in combination. Although this model awaits clinical confirmation, the findings indicate that novel, broadly active mAbs are urgently needed.
SAB Comment: The result of this lab study parallels clinical evidence of waning efficacy of monoclonal antibodies, including Evusheld, among patients exposed to omicron BQ.1.1 subvariants, currently responsible for roughly 50% of US infections. The study does not consider secondary resistance conferred by infection or vaccination. - Platelet dysfunction and thrombus instability in flow conditions in patients with severe COVID-19. 11/14/2022. Tacquard C. Thromb Res.
Between April 2020 and June 2021, 35 ICU patients with COVID-19 had serial blood samples taken at days 0, 2 and 7. These were examined for changes in platelet function with a variety of detailed tests. The results of the testing suggest: 1. Platelets from patients with severe COVID-19 show signs of pre-activation at ICU admission. 2. Platelets from patients with severe COVID-19 are hyporeactive to several agonists. 3. Platelet hyporeactivity may be related to platelet exhaustion or to a plasmatic factor. 4. Platelets from patients with severe COVID-19 form smaller and unstable thrombi. These data help further characterize the clotting and bleeding that occurs with COVID-19. However, these data may or may not apply to the current variants.
SAB Comment: This small pilot study does add to the understanding of platelet dysfunction for ICU patients with COVID-19. However, these preliminary data do not change the clinical management of these patients. - Trends in Clinician Burnout With Associated Mitigating and Aggravating Factors During the COVID-19 Pandemic. 11/23/22. Linzer M. JAMA Health Forum.
Presented are the results of 204 ongoing surveys regarding burnout administered to 56,000 physicians and advanced caregivers by members of the AMA partner healthcare systems, between February 2019 and December 2021, with 21,000 responses. Using the Mini Z work-life measure, topics included clinician outcomes (stress, satisfaction, burnout and intent to leave), and mitigating or aggregating factors (feeling valued, teamwork, work pace, work control, chaos, electronic health record factors, time pressure, and values alignment). Burnout occurred with 45% of respondents in 2019 and by late 2021, it was 60%. Intent to leave rose from 24% to greater than 40%. Mitigating factors that reduced burnout significantly were a calm vs chaotic environment 42%, good work control 36%, good teamwork 39% and feeling valued 32%. The authors concluded that monitoring and improving factors of providers’ working conditions may aid workforce retention. - Twice-Daily Oral Zinc in the Treatment of Patients With Coronavirus Disease 2019: A Randomized Double-Blind Controlled Trial. 11/11/22. Ben Abdallah S. Clin Infect Dis.
This prospective, randomized, double-blind, placebo-controlled, multicenter trial included both COVID-19 positive outpatients (n= 190) and inpatients (n= 280). Patients received 25mg of oral zinc (Zn) twice a day or placebo for 15 days. Mortality was 6.5% in the Zn group vs. 9.2% in the placebo group (OR 0.68, p=0.27, not statistically significant). ICU admission was 5.2% in the Zn group vs. 11.3% for the placebo group (OR 0.43. p=0.01). For inpatients, hospital length of stay was significantly shorter in the Zn group vs. the placebo group by 3.5 days. In outpatients, the duration of symptoms decreased by 1.9 days amongst the Zn treated group. Consistent results were observed in subgroups of patients based on age, comorbidities and oxygen therapy.
SAB Comment: The article didn’t mention serum Zn levels, so we cannot comment as to whether Zn prescription was for a Zn deficiency or used as a supplement. Larger studies should be done before Zn is considered a magic bullet for COVID-19 infection.
November 21, 2022:
 Acute and postacute sequelae associated with SARS-CoV-2 reinfection. 11/10/22. Bowe B. Nat Med.
Acute and postacute sequelae associated with SARS-CoV-2 reinfection. 11/10/22. Bowe B. Nat Med.
This US Veterans Health Administration (VHA) study quantitates the health burdens of reinfection with SARS-CoV-2. Using the extensive VHA database, the outcomes for over 5 million people were compared between March 2020 and April 2022. The authors compared the cohorts with one infection (n=443,588), with two or more infections (n=40,947) and with no infection (n=5,334,729), during the acute illness and up to 6 months later. Compared to single infection, reinfection contributed additional risks of death (hazard ratio (HR) = 2.17), hospitalization (HR = 3.32) and sequelae in multiple organ systems. Evident regardless of vaccination status, these risks were most pronounced in the acute phase but persisted at six months. Compared to noninfected controls, cumulative risks and burdens of repeat infection increased according to the number of infections.
SAB Comment: This is a sobering picture of repeated infections. As variants evolve, immunity wanes and public health measures change, reinfection can result in major health problems, even for the vaccinated. Previous infection and vaccinations may reduce, but do not prevent COVID hospitalization, mortality and sequelae, including long COVID. The study is not designed to compare the outcomes of initial vs. subsequent infections, but it documents the potential seriousness of reinfection, and highlights the need for strategies to prevent reinfection.- Association between vitamin D supplementation and COVID-19 infection and mortality. 11/13/2022. Gibbons JB. Sci Rep.
Exploring whether vitamin D reduces the risk of COVID-19 infection, this retrospective data mining study compared the incidence of SARS-Co2 infection and Covid-19 mortality in large cohorts of US veterans receiving either vitamin D supplementation before the pandemic (Jan. 2019 – Dec. 2020), during the early stages of the pandemic (March 2020 – Dec. 2020) or not at all. Patients receiving Vitamin D2 and D3 prescriptions experienced reductions in COVID-19 infection of 28% and 20%, respectively [(D3 Hazard Ratio (HR)_=_0.80, [95% CI 0.77, 0.83]), D2 HR_=_0.72, [95% CI 0.65, 0.79] and reductions in mortality within 30-days of COVID-19 infection of 33% with Vitamin D3 and 25% with D2 (D3 HR_=_0.67, [95% CI 0.59, 0.75]; D2 HR_=_0.75, [95% CI 0.55, 1.04]). Subgroup analyses revealed a greater reduction in infection rates among Black patients receiving vitamin D supplements and noted greatest responses in patients with the lowest vitamin D serum levels. The study authors acknowledge several limitations related to methodology of data capture and point towards randomized trials to further validate their results.
SAB Comment: There are a number of controlled trials validating the observations made in this study and vitamin D’s effectiveness as an immunomodulator is well documented. We note that important study limitations include a lack of data on Vitamin D levels, and analysis based only on prescribing information. There is no information on whether subjects actually took either over-the-counter Vitamin D or the preparations prescribed by the VA. - Effect of vitamin D3 supplementation on cellular immunity and inflammatory markers in COVID-19 patients admitted to the ICU. 11/4/2022. Bychinin MV. Sci Rep.
In this single center, double-blind randomized, placebo-controlled pilot trial, 110 ICU patients with severe COVID-19 were enrolled beginning in October 2021 and studied to determine the efficacy of vitamin D3 supplementation (60,000 IU/weekly followed by daily 5000 IU) vs. placebo on cellular immunity, inflammatory markers and clinical outcome. Treatment resulted in increased lymphocyte counts, natural killer and natural killer T cell counts, lower neutrophil-to-lymphocyte ratio, and lower serum levels of inflammatory markers on the 7th day of treatment but no difference in clinical outcomes. The study includes an assessment of the role of vitamin D in immune modulation and explores available literature. - Prevalence and Correlates of Long COVID Symptoms Among US Adults. 10/27/2022. Perlis RH. JAMA Netw Open.
As a part of a large, 50-state, multi-wave general health survey performed between February 2021 and July 2022, these authors identified 16,091 respondents who had suffered a Covid-19 infection as verified by a positive PCR or antigen test. From this cohort, 2359 individuals (14.7%) reported continued COVID-19 symptoms more than 2 months after acute illness which was the definition used here for long Covid. The range and frequency of symptoms reported was similar to previous studies. Long Covid occurred somewhat less frequently when Omicron variants were predominant than when the other named variants were predominant. Completion of the primary vaccine series prior to acute illness was associated with diminished risk for long COVID (OR, 0.72). - Variant-specific symptoms of COVID-19 in a study of 1,542,510 adults in England. 11/11/2022. Whitaker M. Nat Commun.
Investigators used ~monthly data from the large community-based REal-time Assessment of Community Transmission _1 (REACT-1) study to describe the symptom profiles of the dominant SARS-CoV-2 variants in England from 5/1/2020 to 3/31/2022, (Alpha, Delta, Omicron BA.1 and BA.2), and to identify symptoms most predictive of high viral load, indicating higher infectiousness, for each. Using a list of 26 symptoms, documented infection with each successive variant was increasingly likely to be symptomatic. Early variants were most likely to present with loss of smell or taste while Omicron variants most frequently presented with cold/flu like symptoms that were associated with the lowest adjusted cycle threshold values. - Whole Health System Approach to Long COVID. 8/1/2022. Veterans Administration.
This guide describes a whole health system approach to long COVID. It is a PACT (patient-aligned care team) guide from the US Department of Veteran Affairs. The whole health system approach is evidence-informed, multidisciplinary, personalized, and veteran-driven. The guide includes a definition of long COVID and post COVID conditions, current incidences of conditions and recommendations for evaluation and treatment. The guide is meant to be updated periodically.
November 7, 2022:
- Discovery of SARS-CoV-2 antiviral synergy between remdesivir and approved drugs in human lung cells. 11/3/22. Nguyenla X. Sci Rep.
In this basic science study, these investigators conducted combinatorial high-throughput screening to find synergy of approved drugs with remdesivir to prevent SARS-CoV-2 replication in human lung epithelial cells. Of 20 approved drugs found, the strongest synergies were with the hepatitis C virus (HCV) NS5A inhibitors, velpatasvir and elbasvir, which may act on the SARS-CoV-2 exonuclease proofreader. These anti-HCV drugs are commercially available in combination with “partner” drugs, Epclusa® (velpatasvir/sofosbuvir) and Zepatier® (elbasvir/grazoprevir). When velpatasvir and elbasvir were combined with their “partner” drugs, it boosted remdesivir’s potency more than 25-fold. Further fine-tuning the potency and pharmacokinetic properties of these FDA-approved HCV therapeutics, used in combination with remdesivir, could merit clinical testing.
SAB Comment: As SARS-Co-V-2 continues to mutate, novel therapeutics will be crucial. In other viral illnesses (e.g., HIV), drug combinations yield benefits far beyond their constituents used alone. Unexpectedly, these investigators reported HCV drugs targeting a non-mutated part of the virus, the exonuclease proofreader, provide a remarkable boost to remdesivir concentrations achievable in vivo. However, much more work is needed before clinical trials can be considered. The in vitro concentrations required of the HCV drugs may not be achievable in patients and the expense of the HCV drugs may limit their usefulness. The authors also reported common generic drugs that appear to synergize with remdesivir in vitro. We await further testing.  Effectiveness of Evusheld in Immunocompromised Patients: Propensity Score-Matched Analysis. 10/31/22. Najjar-Debbiny R. Science Trans Med.
Effectiveness of Evusheld in Immunocompromised Patients: Propensity Score-Matched Analysis. 10/31/22. Najjar-Debbiny R. Science Trans Med.
This is a real-world efficacy assessment of preexposure prophylactic intramuscular Evusheld administered to a cohort of immunosuppressed patients belonging to Israel’s largest healthcare system. On February 15, 2022, 703 patients were identified to be eligible and received 300 mg of the two-component monoclonal antibody (150 mg of tixagevimab and 150 mg of cilgavimab). This group was propensity matched with a 2,812 patient control group and followed through June 30 or up to 90 days focusing on COVID-19 related illness and hospitalization.
Results identified a reduced risk for SARS-CoV-2 infection and COVID-19-related hospitalization with a hazard ratio of 0.75 and 0.41, respectively. The reasons for the less promising results in this study compared to other recent studies were discussed. The need of a dosage increase, particularly for obese patients, was discussed along with an alert to expect lower responses as new variants emerge.
SAB Comment: This study parallels another previously reviewed paper highlighting Evusheld’s role in preexposure prophylaxis. In addition, a recent CDC fact sheet lists indication, timing and use for immunocompromised patients and those unable to be vaccinated. A higher 300 mg dose of each component (600 mg total) is to be repeated every 6 months where indicated and efficacy may decrease as variants evolve.- Mental health and well-being of anaesthetists during the COVID-19 pandemic: a scoping review. 10/31/2022. Paterson E. Anaesthesia.
Much has been written about the psychological stresses on healthcare providers during the SARS-CoV-2 pandemic. This paper reviewed 20 studies which included 8680 anesthetists from January 2020 to May 2022, to summarize the mental health of anesthesia providers. The papers included providers from many countries, though over half were from the USA. Most of the information relied on self-reported surveys. As with other specialties, burnout, stress, anxiety, depression, post-traumatic stress and insomnia were common. Risks for such problems were female sex, non-white ethnicity and LGBTQIA+. Protective factors included positive job satisfaction, support from family, colleagues and hospital management, older age and male sex. Specific impacts on mental health and well-being were very heterogeneous across countries and practice contexts. - The Next Next Wave: How Critical Care Might Learn From COVID in Responding to the Next Pandemic. 11/1/22. Tung A. Anesth Analg.
The Open Mind is an Anesthesia & Analgesia forum for “…proposing new approaches or solutions to an important issue facing the anesthesiology community.” In this article, “front-line” anesthesiology intensivists were asked how the critical care response to the next pandemic might be informed by their experience during COVID-19. Each section reflects provider experience at different institutions, as follows: (1) managing hospital-wide critical care response to a new disease (NYU Langone Health); (2) converting operating rooms to ICUs (New York Presbyterian-Columbia); (3) evolving airway management of the COVID patient (University of Chicago); (4) adapting extracorporeal membrane oxygenation to COVID-associated acute respiratory failure (Emory University) and (5) the impact of COVID on provider wellness (Beth Israel Deaconess). Lessons learned include that the rapid, unpredictable evolution of pathogens requires nimble adaptability, collaboration and discovery; that published clinical guidance may lag behind empiric adaptation; and that provider stress must be recognized and supported.
SAB Comment: This is a unique overview based on the first-hand experience and contributions of anesthesiology intensivists over the almost three years of the COVID-19 pandemic. Given the pandemic’s enormous physical, emotional, economic and political toll, the lessons learned are germane to all intensivists, regardless of specialty, as well as hospital administrators and leadership. Readers are encouraged to also review the superb overview provided by the accompanying editorial.  Wellness and Coping of Physicians Who Worked in ICUs During the Pandemic: A Multicenter Cross-Sectional North American Survey. 10/27/22. Burns KEA. Crit Care.
Wellness and Coping of Physicians Who Worked in ICUs During the Pandemic: A Multicenter Cross-Sectional North American Survey. 10/27/22. Burns KEA. Crit Care.
This survey supported by the American Thoracic Society assessed ICU attending physician wellness and coping during the COVID-19 pandemic. The results reflect a 43% response rate to 1,080 questionnaires, using four validated instruments from physicians in 62 US and Canadian sites, conducted February-April 2021. About 60% reported moral distress (conflict with internal ethical values) and burnout (emotional exhaustion, depersonalization, reduced personal accomplishment). Moral distress, correlated with increased workload (patient volume, days worked, unscheduled in-house night-shifts), was exacerbated by adverse institutional organization, was greater in women and physicians of color, and contributed to a desire to leave their position in a quarter of respondents. Despite this, most physicians experienced moderate professional fulfillment (satisfaction from attaining career goals). The survey revealed four coping profiles: active planning/social interaction (20%), avoidance/ self-distraction (45%), mixed (5%) or infrequent (30%).
SAB Comment: The phenomenon of pandemic-induced healthcare provider burnout is widely understood, but this survey provides new information on the extent and consequence of moral distress among intensivists. It also quantifies different work stress coping mechanisms, although ironically those who used them infrequently had less moral distress and burnout and more professional fulfilment. This group was older and could represent less intense “front-line” activity, a question not addressed by the authors. This point highlights that this survey quantitates problems but merely hints at solutions. Nonetheless, it provides an important database for future studies on interventions to ameliorate moral distress, burnout and work changes among intensivists.
November 4, 2022:
- Clinical phenotypes and outcomes associated with SARS-CoV-2 variant Omicron in critically ill French patients with COVID-19. 10/12/22. de Prost N. Nat Commun.
This prospective, multicenter observational study from France studied 259 patients with severe COVID-19 requiring ICU admission between December 6, 2021 and May 1, 2022. Extensive viral genomic sequencing was done and Delta and several early Omicron subvariants were identified. Mortality at 28 days in Delta and Omicron patients was not significantly different. The clinical phenotype of patients infected with Omicron was different from that in those infected with Delta. Omicron patients were older, frailer with more comorbidities (especially immunosuppression), and had higher severity of illness scores, reflecting more extrapulmonary organ failure. Among Omicron-infected patients, 43% were immunocompromised and these patients had a higher mortality compared with other Omicron-infected patients (47% vs 26%). Most immunocompromised Omicron-infected patients had been vaccinated (86%) but displayed a poor humoral response to vaccination.
SAB Comment: Continued deaths from COVID-19 during the Omicron period suggest that severe Omicron is a problem, even if general population studies suggest Omicron is not as severe as Delta. This study confirms that ICU patients have as poor a prognosis with Omicron as with Delta, and that the elderly, frail and immunocompromised population is particularly vulnerable. People with such risk factors may want to adopt strategies to avoid getting COVID-19 in the first place. - Dehydration is associated with production of organic osmolytes and predicts physical long-term symptoms after COVID-19: a multicenter cohort study. 10/22/22. Hultström M. Crit Care.
This study used 2 observational cohorts of COVID-19 patients (totaling 1217) from Sweden and Quebec during January 2020 to December 2021 to determine the validity of the physiological response to dehydration known as aestivation (which includes muscle breakdown) and its relevance for COVID-19 outcome. Increased estimated osmolality was associated with increased organic (glucose and urea) osmolytes, a decrease in the proportional ionic (Na+, K+) contribution to osmolality, acute kidney injury, invasive mechanical ventilation, mortality and long COVID characterized by weakness. Metabolemic analysis demonstrated the presence of protein breakdown products likely driving increased hepatic production of glucose and urea, and consistent with aestivation. Further studies are needed to characterize the role of dehydration in viral illness.
October 17, 2022:
- Peripartum Outcomes Associated With COVID-19 Vaccination During Pregnancy: A Systematic Review and Meta-analysis. 10/3/2022. Watanabe A. JAMA Pediatr.
The study comprises a meta-analysis of prospective trials and observational studies comparing 81,389 individuals who received at least one COVID-19 vaccination during pregnancy to 255,346 who did not. The primary outcome was neonatal condition. Vaccination was associated with a lower risk of intrauterine fetal demise (OR 0.73) and NICU admission (OR 0.88). There was no difference in preterm delivery, small for gestational age or low APGAR score. The secondary outcome of maternal condition showed a significant reduction of the incidence of COVID-19 (OR 0.46) and no difference in the incidence of cesarean section, chorioamnionitis, or postpartum hemorrhage. - Pregnancy outcomes after SARS-CoV-2 infection in periods dominated by delta and omicron variants in Scotland: a population-based cohort study. 10/10/2022. Stock SJ. Lancet Respir Med.
To better understand the outcomes of SARS-CoV-2 infection in pregnant women during the delta and early omicron surges, 9823 women with COVID-19 at any point in their pregnancy were studied using a national Scottish database. Women infected during the omicron period were less likely to require critical care admission (OR 0.25) and have preterm birth outcomes (OR 0.57) than pregnant women during the delta period. None of the women died, and the numbers of stillbirths and neonatal deaths were insufficient to realistically analyze. The authors conclude that SARS-CoV-2 infection in pregnancy during the early omicron-dominant period was associated with reduced risk of complications compared with the delta-dominant period. - Real-world effectiveness of molnupiravir and nirmatrelvir plus ritonavir against mortality, hospitalisation, and in-hospital outcomes among community-dwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the omicron wave in Hong Kong: an observational study. 10/10/2022. Wong CKH. Lancet.
Retrospective data analysis covering over 1 million Hong Kong Hospital Authority enrolled patients during a 4-month period starting in February 2022. Among 5000 patients receiving molnupiravir and 6,000 nirmatrelvir+ritonavir during the BA.2.2. subvariant omicron wave, effectiveness was demonstrated using matched controls using propensity score (1:10) according to age, sex, date of SARSCoV-2 infection diagnosis, Charlson Comorbidity Index score, and vaccination status. Molnupiravir decreased mortality (HR 0.76) and in-hospital progression of disease (HR 0.57) but not hospital admissions. Nirmatrelvir+ritonavir lowered mortality (HR 0.34) hospital admission (HR 0.76) and hospital progression similar to molnupiravir. This study is one of the first real-world studies exploring the clinical use of oral antivirals during a pandemic wave dominated by the SARS-CoV-2 omicron variant. An editorial comment summarizing this study’s impact is provided by two French public health professionals and accompanies this publication. - SARS-CoV-2 infection drives an inflammatory response in human adipose tissue through infection of adipocytes and macrophages. 9/22/22. MARTÍNEZ-COLÓN GJ. Science Trans Med.
Why obesity worsens COVID-19 outcomes is unknown. In COVID-19 autopsies, these investigators found SARS-CoV-2 RNA in epicardial, visceral, and subcutaneous adipocytes and in a subset of inflammatory adipose tissue-resident macrophages. Viral replication occurred in mature adipocytes, but adipose tissue-infiltrating macrophages synthesized viral components without producing infective virus (“abortive infection”). SARS-CoV-2 initiated an inflammatory response within both the infected macrophages and in bystander pre-adipocytes. Neither adipocytes nor macrophages consistently expresses ACE2 protein, suggesting viral entry by alternative mechanisms. The authors suggest that adipose tissue SARS-CoV-2 infection and induction of local and systemic responses driven by adipose tissue-resident macrophages could contribute to COVID-19 severity.
SAB Comment: This study represents early research that may lead to a better understanding of whether or not processes within adipocytes add to the expected pulmonary compromise of obesity during COVID. Future research is required. - Tracheostomy outcomes in critically ill patients with COVID-19: a systematic review, meta-analysis, and meta-regression. 10/1/2022. Battaglini D. Br J Anaesth.
This review chose 47 articles (n=5268 patients) for analysis. High levels of between-study heterogeneity were observed across study outcomes. The pooled mean tracheostomy timing was 16.5 days. Pooled mortality was 22.1%. Meta-regression did not show significant associations between mortality and tracheostomy timing, mechanical ventilation duration, time to decannulation, and tracheostomy technique. Duration of mechanical ventilation was not associated with tracheostomy timing. Data were insufficient to assess the effect of tracheostomy technique on mechanical ventilation duration.  Use of Cardiopulmonary Exercise Testing to Evaluate Long COVID-19 Symptoms in Adults: A Systematic Review and Meta-analysis. 10/12/22. Durstenfeld MS. JAMA Netw Open.
Use of Cardiopulmonary Exercise Testing to Evaluate Long COVID-19 Symptoms in Adults: A Systematic Review and Meta-analysis. 10/12/22. Durstenfeld MS. JAMA Netw Open.
This systematic review and meta-analysis examines changes in cardiopulmonary exercise testing (CPET) for patients with long COVID (LC). A total of 38 studies were identified that performed CPET on 2,160 individuals 3 to 18 months after SARS-CoV-2 infection, including 1,228 with symptoms consistent with LC. Most studies were case series of individuals with LC or cross-sectional assessments within posthospitalization cohorts. Based on a meta-analysis of 9 studies that compared a total of 464 LC patients with prevalent symptoms to 359 others without, the mean peak V̇O₂ was lowered by 4.9 mL/kg/min in symptomatic individuals. While cardiopulmonary exercise testing is not readily available and there are limitations including selection bias, limited data sets and variability in definitions, this study represents a compilation of the present understanding in this field and helps identify future areas of research.
SAB Comment: As the importance of LC gains attention, this data summarizes prior literature and helps point the way forward. An accompanying podcast in the multimedia link summarizes the article well and adds to the clinical utility of these findings.
October 12, 2022:
- Clinical, Virologic, and Immunologic Evaluation of Symptomatic Coronavirus Disease 2019 Rebound Following Nirmatrelvir/Ritonavir Treatment. 10/6/22. Epling BP. Clin Infect Dis.
This is a highly sophisticated analysis of the post-Paxlovid rebound phenomenon and its clinical significance. NIH investigators used viral sequencing and culture, serologic data, T-cell stimulation assays and soluble biomarkers in an 8-patient cohort of mostly Omicron BA.2 breakthrough infections who exhibited rebound and a 7-patient infected control group who did not.
Robust cytokine-producing, proliferating, activated SARS-CoV-2–specific T-cell responses were greater during rebound than during early acute COVID-19, along with rising T-cell counts. Anti-nucleocapsid IgG and Omicron-specific neutralizing antibodies were also increased in 6 patients with rebound symptoms.
This suggests that clinical rebound may be an indicator of a robust immune response rather than uncontrolled viral replication driving inflammation or a significant risk of impending disease progression. No patient developed severe disease during rebound, and adaptive immunity against SARS-CoV-2 appeared intact. - Correlation between COVID-19 severity and previous exposure of patients to Borrelia spp. 9/24/2022. Szewczyk-Dąbrowska A. Sci Rep.
In this small study, exposure to Borrelia, the causative agent of Lyme disease, was identified by 19-antigen serological testing to be strongly correlated with severity of subsequent COVID-19 disease. Subjects with mild/asymptomatic COVID-19 (n=28), or hospitalization for COVID-19 (n=31), were compared with a group never infected with SARS-CoV-2 (n=28). Increased levels of Borrelia-specific IgGs strongly correlated with COVID-19 severity and risk of hospitalization. All hospitalized patients had IgG markers of Borrelia exposure while around half of the other two groups did. These findings suggest that a history of tick bites and related infections may contribute to COVID-19 risks in unknown ways. - Effectiveness of Molnupiravir in High Risk Patients: a Propensity Score Matched Analysis. 9/21/2022. Najjar-Debbiny R. Clin Infect Dis.
Retrospective health care data analysis to examine real life effectiveness of molnupiravir in Israel between January and February 2022. Focusing on a composite outcome of severe illness or death among 2,000+ patient cohorts with mild to moderately severe Covid-19 and one or more risk factors, irrespective of vaccination status, the authors determined that molnupiravir was associated with a non-significant risk reduction (HR 0.83 [95% CI, 0.57-1.21]) for mortality and severe illness. It did provide a significant incremental benefit in specific subgroups, including older, female and inadequately vaccinated patients and should be considered a viable alternative to nirmatrelvir/ritonavir when dealing with drug interactions and side-effects.  Long-term neurological sequelae of SARS-CoV-2 infection. 10/3/22. Nat Med.
Long-term neurological sequelae of SARS-CoV-2 infection. 10/3/22. Nat Med.
This is a research briefing by the senior author of a number of studies examining long-term sequelae of COVID-19 using the US Department of Veteran Affairs database. It highlights the fact that patients who survive the first 30 days of acute SARS-CoV-2 infection have a 42% increased risk of developing various neurological disorders during the subsequent year compared with uninfected contemporaries. Although the absolute burden of developing any neurological disorder might seem small (7% at 1 year), given the large scale of the pandemic, this translates into a large number of affected individuals. The authors used a cohort of 154,068 infected patients and 5,638,795 uninfected controls in addition to a prepandemic historical control of almost 6 million patients. Risk correlated with acute COVID severity. They emphasize the need for early recognition and treatment instead of dismissal of nonspecific symptoms, provide a number of references, and urge further investigation.
SAB Comment: A more detailed account of this research can be found below.
Long-term neurologic outcomes of COVID-19. 9/22/22. Xu E. Nat Med.- Protection against omicron (B.1.1.529) BA.2 reinfection conferred by primary omicron BA.1 or pre-omicron SARS-CoV-2 infection among health-care workers with and without mRNA vaccination: a test-negative case-control study. 9/24/2022. Carazo S. Lancet Infect Dis.
In an effort to understand vaccine-induced and infection-induced immunity against omicron subvariant BA.2, researchers from Quebec conducted a test-negative case-control study in health-care workers between March 27 and June 4, 2022, when BA.2 was dominant. Subjects were health-care workers who underwent RT-PCR testing a total of 256,636 times during the study period. Positive cases were compared with negative controls using Quebec’s registries of historic RT-PCR results and vaccinations. Previous omicron BA.1 infection without vaccination was the single most protective factor against BA.2 reinfection (risk reduction of 72%) and was associated with higher protection than pre-omicron infection alone (38%) or even than three doses of mRNA vaccine in people with no previous infection (46%). Hybrid immunity conferred by previous omicron BA.1 infection plus vaccination increased estimated protection against BA.2 reinfection to 96% with two or three vaccine doses, and this protection was maintained for at least 5 months after primary infection.
SAB Comment: A previous BA.1 omicron infection, especially with vaccination, is highly protective, and this protection seems to last longer than protection from infection with non-omicron variants. Since the new bivalent mRNA vaccines were developed in part from BA.1, this study provides hope that the bivalent mRNA vaccines may be effective and longer lasting. An accompanying comment details other studies looking at BA.4 and BA.5, which show that the omicron variant has continued to evolve with increasing antibody neutralization escape. - Review: Long COVID in Children
Original Article: Clinical Features and Burden of Postacute Sequelae of SARS-CoV-2 Infection in Children and Adolescents. 10/4/2022. R: Slomski A
O: Rao S. JAMA.
The electronic health records of PEDSnet institutions from 3/2020-10/2021 were used in this assessment of PASC in children. Of 659,286 children tested by PCR, 59,893 were positive for COVID-19. Diagnosis codes for the next 28-179 days were evaluated for all with PCR negative patients as controls. Patients with MIS-C were not included. The most common findings were loss of taste or smell (HR 1.96), myocarditis (HR 3.10), and use of cough and cold preparations. There was at least 1 symptom of PASC in 41.9% of positive cases and in 38.2% of PCR negative controls, with a difference of 3.7%, a much lower incidence than in adults. ICU care, age less than 5, and complex chronic conditions were associated with PASC findings. According to the authors, symptoms more common in children than adults also included abnormal liver enzymes, hair loss, skin rash, and diarrhea. - Waning of vaccine effectiveness against moderate and severe covid-19 among adults in the US from the VISION network: test negative, case-control study. 10/3/2022. Ferdinands JM. BMJ.
Retrospective data from the CDC Vision Network was obtained for this test negative case control study carried out from January 2021-July 2022. The outcome was mRNA COVID-19 vaccine effectiveness for pre-delta, delta, and omicron variant periods among patient groups based on age, 2 month intervals from last vaccine dose, and immunocompromised status. Included were 893,461 patients presenting with COVID-19-like symptoms from 261 hospitals, 272 emergency departments, and 119 urgent care facilities. 45,903 patients hospitalized with positive tests for COVID-19 (cases) were compared to 213,103 test negative controls. Likewise 103,287 patients presenting at emergency and urgent care testing positive were compared to 531,168 test negative controls. As expected, effectiveness against infection and hospital admission waned as time from the last dose increased. The tables permit quick assessment of group data.
October 4, 2022:
 Circulating anti-nuclear autoantibodies in COVID-19 survivors predict long-COVID symptoms. 9/22/22. Son K. Eur Respir J.
Circulating anti-nuclear autoantibodies in COVID-19 survivors predict long-COVID symptoms. 9/22/22. Son K. Eur Respir J.
This longitudinal observational multi-institutional Canadian study of 106 COVID-19 patients from 8/2020 to 9/2021 examined the relationship between long COVID symptoms and antinuclear/extractable-nuclear antibodies (ANA/ENA). Patients, whose acute COVID symptoms varied, were evaluated at 3, 6, and 12 months with ANA/ENA and cytokine assays. They self-reported fatigue, coughing and dyspnea. Controls were age- and sex-matched, nonimmunized, COVID-negative volunteers (22), and non-COVID-19 positive patients with respiratory symptoms (34). At 3 months, ANA levels were increased in COVID patients compared to controls, but decreased over the year. Higher levels of tumor necrosis factor alpha, D-dimer and IL-1β predicted elevated ANA at 12 months. Tracking the increase of antibodies associated with autoimmune disease over time demonstrated potential de novo autoantibody synthesis. The authors conclude that persistently positive ANAs at 12 months post-COVID-19 are associated with continuing symptoms and inflammation in a subset of COVID survivors.
SAB Comment: The authors present elegant immunological evidence of an autoimmune factor in the development of long COVID. However, this small study has much potential for bias. Only 57 of 106 patients were available at 12 months. Also, in a study which concluded severity of disease was important, only 1 of 34 respiratory symptom control patients was hospitalized compared to 80 of 106 COVID-19 patients.- COVID-19 mRNA Vaccine Safety Among Children Aged 6 Months-5 Years – United States, June 18, 2022-August 21, 2022. 9/1/22. Hause AM. MMWR Morb Mortal Wkly Rep.
In the time period noted above, 1,040,230 children received Pfizer-BioNTech or Moderna vaccines. Approximately 23,266 children were enrolled in v-safe, the CDC voluntary cell phone-based monitoring system. The most frequent systemic reactions reported to v-safe after receipt of vaccines were irritability or crying in approximately one half of children aged 6 months–2 years. In children aged 3 years or older, systemic reactions after vaccination were less frequently reported; injection site pain was the most frequently reported reaction among these older children. The VAERS reporting system received a total of 1,017 reports of adverse events after Pfizer-BioNTech or Moderna vaccination, 98.1% of which were classified as nonserious. No reports of myocarditis after vaccination were reported. Health care providers and parents of young children should be aware that local and systemic reactions are expected after vaccination with Pfizer-BioNTech or Moderna vaccine and that serious adverse events are rare. - Emotional Exhaustion Among US Health Care Workers Before and During the COVID-19 Pandemic, 2019-2021. 9/21/2022. Sexton JB. JAMA Netw Open.
This multicenter electronic survey of tens of thousands of clinical and non-clinical hospital-based health care workers was administered September 2019, September 2020, and September 2021 – January 2022. Response rates were 75-85%, amounting to 31,475 – 45,268 per survey. Physicians reporting emotional exhaustion were 32% in 2019, 28% in 2020, and 38% during the second pandemic year. Nurses reported 41%, 47% and 49% respectively. Others showed a similar pattern to nurses, at lower levels. “These findings indicate that emotional exhaustion among health care workers, which was problematic before the pandemic, has become worse; increases in emotional exhaustion may jeopardize care quality and necessitate additional support for the workforce.”
SAB Comment: Readers will find the JAMA Invited Commentary by Shechter and Norful provides a very cogent overview and interpretation of the findings of this study and puts them into the overall context of the pandemic. - One-Year Mental and Physical Health Assessment in Survivors After ECMO for COVID-19-related ARDS. 9/23/2022. Chommeloux J. Am J Respir Crit Care Med.
In this multicenter, prospective comparative study of ICU patients with or without COVID but with ARDS, MV + ECMO & were evaluated for follow-up status at 6 & 12 months for their PFT, physical, psychological and quality of life (QoL). The authors noted PFTs were normal at 6 months except DLCO which also remained partially impaired at 12 months. The authors point to their outcome of physical, mental and emotional health as that remained disturbingly low compared to non-COVID ECMO patients. Staggering issues were noted for QoL: anxiety for 44% and 42% with PTSD/depression for more than 12 months. The authors attributed this poor health for patients to COVID-19 more than to ECMO. Only 32% returned to work compared to 72% with similar comorbidities. - Respiratory Support in the Time of COVID-19. 9/27/22. Nichol AD. JAMA.
This editorial reviews 6 prior randomized studies of non-invasive respiratory support for patients with COVID-19 pneumonitis along with the SOHO-COVID Randomized Clinical Trial, published in the same issue of JAMA. After discussing each study, the authors conclude that “the available evidence suggests that the initial choice of supplemental oxygen therapy for patients with COVID-19-related acute hypoxemic respiratory failure does not influence mortality. This will provide some reassurance for clinicians in times of reduced availability of certain noninvasive respiratory support strategies during surges in COVID-19 hospitalizations.” They found that the rates of intubation using high-flow nasal oxygen vs conventional oxygen therapy did not consistently favor one modality or the other in this group of studies.
September 28, 2022:
- A Bivalent Omicron-Containing Booster Vaccine against Covid-19. 9/16/22. Chalkias S. N Engl J Med.
This industry-sponsored study presents the interim results of Moderna’s phase 2-3 trial of their bivalent mRNA vaccine. Participants (n = 890) who had received a primary series of two doses and a single booster of Moderna’s original vaccine were divided into two groups; one received another booster of the original vaccine, and the other received the new bivalent mRNA vaccine which includes 25mcg of the original, and 25 mcg of an mRNA vaccine developed against Omicron BA-1. At 29 days, the bivalent Omicron-containing vaccine elicited neutralizing antibody responses against Omicron (including BA.4/5 subvariants) and previous variants that were superior to those of the original vaccine, without safety concerns.
SAB Comment: These interim laboratory immune results are encouraging. In the near future we look forward to the important vaccine effectiveness and longevity results. - Impact of the use of oral antiviral agents on the risk of hospitalization in community COVID-19 patients. 8/28/2022. Yip TCF. Clin Infect Dis.
Authors from Hong Kong examined retrospectively records from outpatients presenting In February and March 2022 during an Omicron surge to compare the effectiveness of 2 antiviral drugs, molnupiravir (n=5808) or nirmatrelvir/ritonavir (n= 4921) to a group with noRx (n = 83,154) in reducing hospital admission (primary endpoint) or in reducing a composite of intensive care unit admission, invasive mechanical ventilation use, and/or death (secondary outcome) in a median f/u of 30 days. After propensity score weighting, nirmatrelvir/ritonavir use (weighted hazard ratio 0.79) but not molnupiravir use (weighted hazard ratio 1.17) was associated with a reduced risk of hospitalization compared to non-treated patients. Neither the use of molnupiravir nor the use of nirmatrelvir/ritonavir was associated with a lower risk of the composite secondary endpoint as compared to non-treated patients.
SAB Comment: While this study shows reduced or absent effect of these medications as compared with other contemporary studies, the authors note that prior to their efforts to propensity match the groups, “oral antiviral users were older and had more comorbidities, lower complete vaccination rate, and more hospitalizations in the previous year.” With propensity matching comparison of all the three groups, they were able to reduce biases of the groups but maintain their final inference. - Long-term cardiac pathology in individuals with mild initial COVID-19 illness. 9/5/2022. Puntmann VO. Nat Med.
These authors conducted serial cardiac assessments in selected patients with COVID-19 with no previous cardiac disease or notable comorbidities by measuring blood biomarkers of heart injury and with magnetic resonance imaging. Baseline measurements from 346 individuals with COVID-19 (52% females) were obtained at a median of 109 days after infection, when 73% of participants reported cardiac symptoms. Symptomatic individuals had higher heart rates and higher imaging values or contrast agent accumulation, denoting inflammatory cardiac involvement, compared to asymptomatic individuals. At follow-up (329 mean days after infection), 57% of participants had persistent cardiac symptoms. Diffuse myocardial edema was more pronounced in participants who remained symptomatic at follow-up as compared to those who improved. Female gender and diffuse myocardial involvement on baseline imaging independently predicted the presence of cardiac symptoms at follow-up. While these data help further characterize cardiac long COVID, these cardiac MRIs are not readily available and there is no treatment yet for this cardiac inflammation. - Nirmatrelvir-Ritonavir and Viral Load Rebound in Covid-19. 9/7/22. Anderson AS. N Engl J Med.
Using data from the EPIC-HR3 trial which compared nirmatrelvir-ritonavir (Paxlovid) to placebo in unvaccinated patients, the authors found that viral load rebound following nirmatrelvir-ritonavir is not retrospectively associated with low nirmatrelvir exposure, recurrence of moderate to severe symptoms or development of resistance to nirmatrelvir. During the recruitment period from June to December 2021 which coincided with delta variant predominance, viral load rebound occurred in 23 of 990 patients (2.3%) in the nirmatrelvir– ritonavir group and in 17 of 980 (1.7%) in the placebo group. These results – published in a research letter suggest that viral load rebound may be a natural feature of a small percentage of SARS CoV-2 infections. However, viral load as determined by PCR assay, may not explain the frequently noted clinical syndrome.  Persistent circulating SARS-CoV-2 spike is associated with post-acute COVID-19 sequelae. 9/2/22. Swank Z. Clin Infect Dis.
Persistent circulating SARS-CoV-2 spike is associated with post-acute COVID-19 sequelae. 9/2/22. Swank Z. Clin Infect Dis.
This retrospective pilot study measured 3 SARS-CoV-2 antigens (using a Simoa assay) in blood samples from 63 individuals diagnosed with COVID-19, of whom 37 had postacute sequelae of COVID-19 (PASC) identified. SARS-CoV-2 spike antigen was detected several months postinfection in 60% of PASC and in none with COVID-19 only. Longitudinal blood samples were available in 12 PASC individuals and in 6 with COVID-19 only. Serial blood samples in 12 PASC individuals showed sustained or fluctuating spike antigen levels, whereas in samples of 6 with COVID-19 only, high antigen levels immediately after diagnosis dropped rapidly. The authors indicate that more study is needed and propose these results support the hypothesis that a reservoir of active virus persists within the body in individuals with PASC. Ten cytokines were also measured and were not different among the groups. The discussion further analyzes the validity of the findings.
September 12, 2022:
- Anticoagulation and Antiplatelet Therapy for Prevention of Venous and Arterial Thrombotic Events in Critically Ill Patients with COVID-19: COVID-PACT. 8/29/2022. Bohula EA. Circulation.
The paper examines the use of prophylactic full-dose anticoagulation and antiplatelet therapy in critically-ill COVID-19. This RCT examined full-dose anticoagulation or standard-dose prophylactic anticoagulation. Absent an indication for antiplatelet therapy, patients were additionally randomized to either clopidogrel or no antiplatelet therapy. The primary efficacy outcome was the hierarchical composite of death due to venous or arterial thrombosis, pulmonary embolism, clinically-evident deep venous thrombosis (DVT), type 1 myocardial infarction, ischemic stroke, systemic embolic event or acute limb ischemia, or clinically-silent DVT. 390 patients were randomized between anticoagulation strategies and 292 between antiplatelet strategies. Full-dose anticoagulation, but not clopidogrel, reduced thrombotic complications with an increase in bleeding, and no apparent change in mortality.  Clinical and Genetic Risk Factors for Acute Incident Venous Thromboembolism in Ambulatory Patients With COVID-19. 8/18/22. Xie J. JAMA Intern Med.
Clinical and Genetic Risk Factors for Acute Incident Venous Thromboembolism in Ambulatory Patients With COVID-19. 8/18/22. Xie J. JAMA Intern Med.
The authors examined data from the UK Biobank for ambulatory patients from March 1, 2020 to September 3, 2021. In this retrospective cohort study of 18,818 outpatients with COVID-19 and 93,179 propensity score-matched noninfected participants, a higher venous thromboembolism (VTE) incidence was observed in the former (hazard ratio, 21.42 (!)); however, this risk was considerably attenuated among the fully vaccinated, after breakthrough infection (hazard ratio, 5.95). Older age, male sex, obesity, no vaccination or partial vaccination, and inherited thrombophilia were independent risk factors for COVID-19–associated VTE. These data help identify high-risk patients for VTE in the ambulatory setting. However, these data predate the Omicron variant. Comparison of Severe Maternal Morbidities Associated With Delivery During Periods of Circulation of Specific SARS-CoV-2 Variants. 8/12/22. Mupanomunda M. JAMA Netw Open.
Comparison of Severe Maternal Morbidities Associated With Delivery During Periods of Circulation of Specific SARS-CoV-2 Variants. 8/12/22. Mupanomunda M. JAMA Netw Open.
Does the incidence of severe maternal morbidity (SMM) vary according to the SARS-CoV-2 variant? In a retrospective study involving 32 US hospitals from March 2020 to January 2022, the authors evaluated SMM in 3,129 mothers with positive RT-PCR results at the delivery hospitalization compared with a propensity-matched cohort of 12,504 mothers who tested negative. SARS-CoV-2 infection at the time of delivery was significantly associated with SMM in the wild-type, Alpha and Delta periods; the magnitude of the associations was most pronounced during the time period when the Delta variant was predominant (OR 7.69). Respiratory (but not nonrespiratory) SMM were significantly higher during the Omicron period. The authors stress the importance of promoting vaccination acceptance and combating misinformation together with other prevention strategies in pregnant individuals.
SAB Comment: As COVID-19 variants, infection patterns, and vaccines evolve, teasing out risks and outcomes has become more intricate. This study focuses on SARS-CoV-2 infection at the time of delivery and provides important information for this particular cohort. An associated commentary helps put the study in perspective and emphasizes the importance of including pregnant patients in the early clinical trials of vaccine safety and efficacy.- Effect of therapeutic versus prophylactic anticoagulation therapy on clinical outcomes in COVID-19 patients: a systematic review with an updated meta-analysis. 8/23/2022. Duo H. Thromb J.
This analysis of 11 randomized clinical trials (RCTs) and 17 observational studies (OBs) from January 8, 2019, to January 8, 2022 compared prophylactic (P-AC) and therapeutic anticoagulant (T-AC) treatment in 16,167 COVID-19 patients. There was no significant overall mortality difference between T-AC and P-AC. The risk of major bleeding with T-AC was higher in (RCTs: RR 1.8, OBs: RR 2.4). In OBs, mortality following T-AC was lower than P-AC in critically ill patients and higher in the non-critically ill. T-AC reduced the incidence of venous thromboembolism by around half in both RCTs and OBs with little difference in arterial thrombosis. T-AC treatment did not reduce mortality risk patients with high d-dimer levels in RCTs. Discussion follows, including need to individualize treatment. - Management and outcomes of pregnant women admitted to intensive care unit for severe pneumonia related to SARS-CoV-2 infection: the multicenter and international COVIDPREG study. 8/19/22. Péju E. Intensive Care Med.
The object of this retrospective multicenter international study from France was to assess ventilatory management, as well as obstetric management and neonatal outcomes, of all pregnant women (187) admitted to 32 ICUs with SARS-CoV-2 pneumonia from March 2020 through December 2021. Outcomes were compiled according to trimester of pregnancy, comorbidities, degree of CT scan abnormalities and mode of ventilatory support, among others. Prone positioning was used in 26% of all patients and 56% of intubated patients, but to a lesser degree before delivery. In the 27 intubated, ventilated patients for whom complete data were available, delivery of the neonate significantly improved oxygenation and decreased driving pressure, with a trend toward increased compliance and decreased plateau pressure. Venovenous ECMO was used in 21% of intubated patients. Maternal mortality was 2% and neonatal mortality 4%. Preterm birth occurred in 74% and ICU admission in 54% of neonates of intubated patients. - Neurological and psychiatric risk trajectories after SARS-CoV-2 infection: an analysis of 2-year retrospective cohort studies including 1 284 437 patients. 8/20/22. Taquet M. Lancet Psychiatry.
This retrospective analysis tracks 14 post-COVID neurologic and psychiatric complications in 3 different age groups and the effect of SARS-CoV-2 variants on those complications between January 2021 and April 2022. Using TriNet X, a US based clinical research collaboration platform deployed by a federated network of 62 health care organizations and tracking 89 million anonymized electronic medical record patient data globally, enabled comparison with a propensity matched control group with other non-COVID related respiratory infections. Results indicate that trajectories for mood and anxiety disorders trend lower overall but cognitive defects, dementia, psychotic disorders, and epilepsy remain elevated 2 years after SARS CoV-2 infection among adults, while children are less susceptible overall except for epilepsy. During the Omicron phase of the pandemic, despite less severe disease and lower mortality, neurologic and psychiatric complications continued unabated and pose a considerable future challenge to health care systems.
In an editorial, the use of comparison groups is highlighted as adding valuable perspective to the multifaceted post-Covid symptom complex and prospective studies to validate the results are recommended. - Prognostic value of von Willebrand factor and ADAMTS13 in patients with COVID-19: A systematic review and meta-analysis. 8/26/2022. Xu X. Thromb Res.
The authors reviewed the current literature and performed meta-analyses on the relationship between both VWF and its cleaving protease ADAMTS13 with the prognosis of COVID-19. The unfavorable outcomes were defined as mortality, intensive care unit (ICU) admission, and severe disease course. A total of 3764 patients from 40 studies were included. The estimated pooled means indicated increased plasma levels of VWF:Ag, VWF:Rco, and VWF:Ag/ADAMTS13:Ac ratio, and decreased plasma levels of ADAMTS13:Ac in COVID-19 patients with unfavorable outcomes when compared to those with favorable outcomes (composite outcomes or subgroup analyses of non-survivor versus survivor, ICU versus non-ICU, and severe versus non-severe). The imbalance of the VWF-ADAMTS13 axis (massive quantitative and qualitative increases of VWF with relative deficiency of ADAMTS13) is associated with poor prognosis of patients with COVID-19. However, the authors conclude that there are currently insufficient data available to recommend VWF-related variables as routine clinical assessments. - Real-world effectiveness of early molnupiravir or nirmatrelvir-ritonavir in hospitalised patients with COVID-19 without supplemental oxygen requirement on admission during Hong Kong’s omicron BA.2 wave: a retrospective cohort study. 8/27/2022. Wong CKH. Lancet Infect Dis.
This retrospective cohort study aimed to evaluate the clinical and virological outcomes associated with molnupiravir (Lagevrio) or nirmatrelvir–ritonavir (Paxlovid) use in hospitalised patients with mild-to-moderate COVID-19 during a pandemic wave dominated by the omicron BA.2 subvariant. A lower risk of all-cause mortality was observed in molnupiravir recipients (HR 0.48) and in nirmatrelvir–ritonavir recipients (HR 0.34) compared to propensity matched controls. Oral antiviral recipients also had lower risks of the composite disease progression outcome (molnupiravir HR 0.60; nirmatrelvir–ritonavir 0.57) and need for oxygen therapy (molnupiravir 0.69; nirmatrelvir–ritonavir 0.73 compared with controls. - The Association of Baseline Plasma SARS-CoV-2 Nucleocapsid Antigen Level and Outcomes in Patients Hospitalized With COVID-19. 8/29/2022. ACTIV-3/TICO Study Group*. Ann Intern Med.
This study examined baseline plasma COVID-19 nucleocapsid antigen level from 2540 patients from August 2020 to November 2021.This was performed at 114 centers in 10 countries in adults hospitalized for acute SARS-CoV-2 infection. Baseline pulmonary severity of illness was strongly associated with plasma antigen level, with mean plasma antigen level 3.10-fold higher among those requiring noninvasive ventilation or high-flow nasal cannula compared with room air. Plasma antigen level was higher in those who lacked antispike antibodies (6.42 fold) and in those with the Delta variant (1.73 fold). Additional factors associated with higher baseline antigen level included male sex, shorter time since hospital admission, decreased days of remdesivir, and renal impairment. In contrast, race, ethnicity, body mass index, and immunocompromising conditions were not associated with plasma antigen levels. Plasma antigen level of 1000 ng/L or greater was associated with a higher odds of worsened pulmonary status at day 5 (odds ratio, 5.06) and longer time to hospital discharge.
August 29, 2022:
- Levels of SARS-CoV-2 antibodies among fully vaccinated individuals with Delta or Omicron variant breakthrough infections. 8/1/22. Breinholt Stærke N. Nature Communications.
Using the Danish National Microbiology database, this observational cohort study examined whether levels of anti-spike IgG influenced rates of Delta and Omicron breakthrough infections in a fully vaccinated cohort. Anti-spike IgG levels were measured before vaccinations and at days 21–28, 90, 180 post first-vaccination, and 4 weeks post-booster. Among 6076 participants, Delta and Omicron (BA.2) infections occurred in 127 and 364, respectively. The highest vs. lower quintile of anti-spike IgG led to a 71% lower Delta breakthrough infection rate (p = 0.0002). However, no significant quintile differences were observed for Omicron suggesting that the level of anti-spike IgG has limited impact on Omicron breakthrough risk.
SAB Comment: Prior to Omicron’s emergence, and consistent with the Delta variant data in the above study, increasing levels of anti-spike IgG were associated with reduced risk of breakthrough infections. This observation is not valid with Omicron breakthrough infection and does not address T- and B-cell immune function post-vaccination. - Nirmatrelvir Use and Severe Covid-19 Outcomes during the Omicron Surge. 8/24/22. Arbel R. N Engl J Med.
This observational, retrospective, Israeli study of ambulatory patients, conducted January 9, 2022 – March 31, 2022 during the Omicron period, investigated early use of nirmatrelvir, a protease inhibitor. Of 105,352 eligible patients (over 40 yrs of age and considered “high risk”), 3,902 received the drug. The authors report significantly lower rates of hospitalization and death within 35 days in patients over 65 yrs who received nirmatrelvir (HR 0.27) irrespective of previous immunity status. They also noted a further reduction of hospitalization in patients over 65 who had previous immunity to COVID from vaccination, previous infection or both, and received the drug. No benefit of use was noted in patients less than 65 years of age. - Oral Nirmatrelvir and Ritonavir in Non-hospitalized Vaccinated Patients with Covid-19. 8/20/22. Ganatra S. Clin Infect Dis.
Though Paxlovid (nirmatrelvir plus ritonavir, NMV-r) reduces progression to severe disease in high-risk, nonhospitalized unvaccinated patients, benefits among vaccinated patients are unclear. This retrospective cohort study between December 2021 and April 2022 evaluated vaccinated adults (over 18 years), who subsequently developed COVID-19, comparing 1,130 who took NMV-r within five days of diagnosis with an equal number who did not. All-cause emergency room visits, hospitalization, or death within 30 days occurred in 89 patients (7.87%) in the NMV-r cohort, compared to 163 patients (14.4%) in the non-NMV-r cohort (p<0.005), a 45% relative risk reduction. Complications and overall resource utilization (e.g., diagnostic tests) were also decreased.
August 22, 2022:
- An Antibody from Single Human VH-rearranging Mouse Neutralizes All SARS-CoV-2 Variants Through BA.5 by Inhibiting Membrane Fusion. 8/11/22. Luo S. Science Immunology.
Anti-spike-directed vaccines and therapeutic antibodies are increasingly ineffective. Using a humanized mouse model, laboratory investigators inserted human gene segments that promoted mouse B-cells to produce humanized antibody repertoires using “V(D)J recombination,” like humans build antibodies after antigen exposures. Novel anti-spike antibodies were then produced following ancestral spike protein exposure. One antibody, SP1-77, neutralized Alpha, Beta, Gamma, Delta, and all Omicron strains. Though binding to the RBD, SP1-77 does not block ACE2 receptor binding. Shown using groundbreaking imaging, SP1-77 works through a novel downstream mechanism, preventing fusion of virus outer membranes with target cell membranes inhibiting a thus far unmuted RBD site.
SAB Comment: The SP1-77 antibody anti-fusion mechanism may provide insights to future vaccine strategies. In this preclinical study, the humanized “VH1-2/Vκ1-33-rearranging mouse model” yielded diverse human heavy and light chain rearrangements and resultant novel immunoglobulins. Application of these experimental techniques can lead to therapeutic antibody repertoire discoveries against future SARS-Co-V variants or any pathogen (e.g., HIV-1, influenza). - Association of Blood Viscosity With Mortality Among Patients Hospitalized With COVID-19. 7/21/2022. Choi D. J Am Coll Cardiol.
Coronavirus disease-2019 (COVID-19) is characterized by a dysfunctional immune response and abnormal blood rheology that contribute to endothelial dysfunction and thrombotic complications. Whole blood viscosity (WBV) is a clinically validated measure of blood rheology and an established predictor of cardiovascular risk. Authors investigated association between estimated BV (eBV) and mortality among 5,621 hospitalized COVID-19 patients (01/20-11/21). Among hospitalized COVID-19 patients, increased eBV is significantly associated with higher mortality suggesting that eBV can prognosticate patient outcomes in earlier stages of COVID-19. The variables to calculate eBV (hematocrit, albumin, and total protein) are easily obtained from most admission labs, suggesting a possible use of eBV as an efficient and simple risk assessment of patients with COVID-19 to offer proper preventive therapy. - Baricitinib in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial and updated meta-analysis. 7/31/2022. RECOVERY Collaborative Group. Lancet.
Baricitinib significantly reduced the risk of death in 8156 patients hospitalized between February and December 2021 with COVID-19 and studied as part of the ongoing RECOVERY trial. The size of benefit was smaller compared to 8 previous trials (13% vs. 43% reduction in proportional mortality with baricitinib). The total randomized evidence to date suggests that JAK inhibitors (chiefly baricitinib) reduce mortality in patients hospitalized for COVID-19 by about one-fifth.
SAB Comment: A comprehensive resource paper on JAK inhibitors and COVID-19. - BNT162b2 Vaccine Effectiveness against Omicron in Children 5 to 11 Years of Age. 6/29/2022. Cohen-Stavi CJ. N Engl J Med.
This Israeli study finds that as omicron was becoming the dominant variant, two doses of the BNT162b2 (Pfizer) vaccine provided moderate short-term protection against documented SARS-CoV-2 infection and symptomatic Covid-19 in children 5 to 11 years of age. This observational cohort study compared the incidence of PCR positivity and symptomatic Covid-19 in 94,728 vaccinated children matched with unvaccinated controls from late November 2021 to January 7, 2022. The vaccine effectiveness against PCR positivity was 51% and against symptomatic infection was 48% after two doses.
SAB Comment: These findings contrast to the trials of this vaccine during the Delta variant predominance, which found an efficacy of 91% against symptomatic infection in children of this age. - C reactive protein utilisation, a biomarker for early COVID-19 treatment, improves lenzilumab efficacy: results from the randomised phase 3 ‘LIVE-AIR’ trial. 7/6/2022. Temesgen Z. BMJ.
This randomized, blinded, controlled phase three LIVE-AIR trial examined effects of lenzilumab an anti-GM-CSF monoclonal antibody under development, on survival without ventilation (SWOV). Hospitalized patients (n=520) between May 2020 and January 2021, with RA SpO2 ≤94% or requiring supplemental oxygen but not IMV, were given lenzilumab or placebo along with corticosteroids and remdesivir on day 0. In this post-hoc analysis, with baseline CRP <150 mg/L, SWOV, the primary endpoint, through day 28, was achieved in 90% lenzilumab and 79% placebo (p=0.0009), but not with CRP ≥150 mg/L, (p=0.9058). The greatest effects were observed with a medium CRP below 79 mg/L. Adverse events with lenzilumab were comparable to placebo.  COVID-19 and Acute Neurologic Complications in Children. 8/11/22. Antoon JW. Pediatrics.
COVID-19 and Acute Neurologic Complications in Children. 8/11/22. Antoon JW. Pediatrics.
This study is based upon data provided to the Pediatric Health Information System database from 52 pediatric academic centers during a 2-year period beginning March 2020. It focused on prevalence, risk factors and outcomes associated with neurological complications in hospitalized children. Among 15,137 children aged 2 months to 18 years, the overall incidence of acute neurological complication was 7%. Febrile seizures, nonfebrile seizures, and encephalopathy combined accounted for 85% of neurologic complications. Identified risk factors included pre-existing chronic neurologic conditions, older age, race, and ethnicity. Lower odds occurred during the period of Delta variant dominance, and in patients treated with remdesivir and dexamethasone. Neurologic complications were associated with increased mortality (1.8% vs. 0.6%, p<0.001), ICU admission, longer hospitalization, and higher cost of care. These results are comparable to children hospitalized with influenza and other viral illnesses and emphasize the importance of COVID-19 immunization, especially in a high-risk population.- Effectiveness of mRNA COVID-19 vaccines against Omicron and Delta variants in a matched test-negative case-control study among US veterans. 8/3/22. Young-Xu Y. BMJ Open.
This study used VA electronic health record data from 114,640 veterans who had a SARS-CoV-2 test during November 2021 or January 2022 to calculate estimates of vaccine effectiveness and considered November infections to be due to the Delta variant and January infections to be due to the Omicron variant.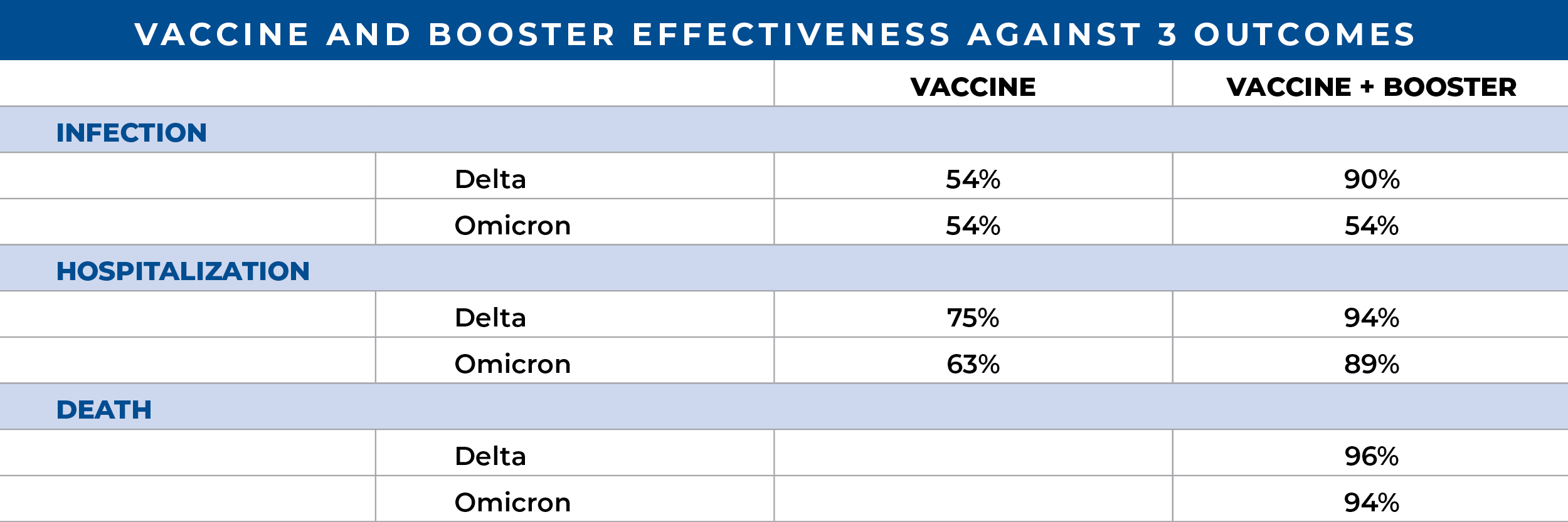
The authors concluded that although the effectiveness of booster vaccination against infection was moderately higher against Delta than against the early Omicron SARS-CoV-2 variant, effectiveness against severe disease and death was similarly high against both variants.  Good practice statements for antithrombotic therapy in the management of COVID-19: Guidance from the SSC of the ISTH. 7/29/22. J Thromb Haemost. Clin Infect Dis.
Good practice statements for antithrombotic therapy in the management of COVID-19: Guidance from the SSC of the ISTH. 7/29/22. J Thromb Haemost. Clin Infect Dis.
This article is a set of best practice statements for deep vein thrombosis prophylaxis and treatment with COVID-19 from the International Society of Thrombosis and Hemostasis. It is not based upon a systematic review but expert opinion. While much of this is similar to prior guidelines, the special considerations section covering pregnancy, pediatrics, chronic kidney disease, obesity and heparin-induced thrombocytopenia without thrombosis are of value.- ISTH guidelines for antithrombotic treatment in COVID-19. 7/29/22. Schulman S. J Thromb Haemost.
This article is a systematic review and guidelines for deep vein thrombosis prophylaxis with COVID-19 from the International Society of Thrombosis and Hemostasis. It was performed by a panel of experts. Among noncritically ill patients hospitalized for COVID-19, the panel gave a strong recommendation (a) for use of prophylactic dose of low molecular weight heparin or unfractionated heparin (LMWH/UFH) (COR 1); (b) for select patients in this group, use of therapeutic dose LMWH/UFH in preference to prophylactic dose (COR 1); but (c) against the addition of an antiplatelet agent (COR 3). Nine additional recommendations are made. The review is comprehensive and current, and the recommendations are graded. Figure 2 is useful as it summarizes the findings. - Long COVID: which symptoms can be attributed to SARS-CoV-2 infection? 8/7/22. Brightling CE. Lancet.
This brief comment places into perspective a long COVID study by Ballering et al, and provides a nutshell view of long COVID. The Ballering study found a long COVID incidence of 12.7% in a geographically and ethnically limited study that did not consider mental health. It also introduces a possible core symptom set for diagnosis. The authors consider the methodology a “major advance on previous long COVID prevalence estimates.” Important concepts introduced in the comment include: “clustering of patient-reported outcomes has identified different severity groups of long COVID and identified increased systemic inflammation in people with very severe long COVID,”; “the proportion of newly infected people developing long COVID is reduced in people who have received vaccination before SARS-CoV-2 infection,” and ”might be lower in people infected with the omicron variant than those infected with earlier variants.” “Current evidence supports the view that long COVID is common and can persist for at least 2 years after SARS-CoV-2 infection, although severe debilitating disease is present in a minority.” - Prognosis and persistence of smell and taste dysfunction in patients with covid-19: meta-analysis with parametric cure modelling of recovery curves. 7/27/2022. Tan BKJ. BMJ.
This metanalysis of COVID positive ve patients (N= 3699) that included 18 retrospective & prospective studies led to a reconstructed parametric “Cure” based model of time-to-event for individual patient data (IPD) on self-reported smell and taste dysfunction. The authors estimated 5.6% could develop a long-term health care burden for those symptoms. In this metaanalysis of IPD, estimated 74%, 86%, 90%, and 96% of patients self-reported smell recovery and 79%, 88%, 90%, and 98% self-reported taste recovery on day number 30, 60, 90, and 180 respectively. Females were associated with poorer recovery of both smell and taste. - Respiratory indications for ECMO: focus on COVID-19. 8/9/2022. Supady A. Intensive Care Med.
This manuscript reviews the world literature for the use of ECMO with COVID-19. The authors point out deficiencies in the literature and possible areas of future research. The authors conclude that during the pandemic, mortality of patients supported with ECMO has increased. However, the precise reasons for this observation are unclear. Known risk factors for mortality in COVID-19 and non-COVID-19 patients are higher patient age, concomitant extra-pulmonary organ failures or malignancies, prolonged mechanical ventilation before ECMO, less experienced treatment teams and lower ECMO caseloads in the treating center. - Risk of SARS-CoV-2 Acquisition in Health Care Workers According to Cumulative Patient Exposure and Preferred Mask Type. 8/15/22. Dörr T. JAMA Netw Open.
In this study of 2,919 Swiss healthcare workers over a 1-year period beginning September 2020, the incidence of SARS-CoV-2 positivity was correlated with cumulative hours of exposure to patients with COVID-19 and lower with exclusive use of FFP2 respirator masks (similar to N95) vs. surgical/mixed mask use. Positivity rates (by testing and/or antinucleocapsid Ab seroconversion) was 13% for workers with no patient contact, 21% for workers caring for COVID patients using respirator masks exclusively and 35% for workers who used a combination of masks. Other significant factors were positive household contacts (OR 7.8), and vaccination (OR 0.55), but not working in ICU.
SAB Comment: Although this study has weaknesses, including self-reported data, we have seen few others reporting the relative benefits of respirator mask wearing. Given the current availability of respirator masks to consumers along with the high rate of breakthrough infections of the very infectious BA.5 Omicron variant, these data may be useful to all who wish to optimize COVID protection in crowded, indoor, and travel environments in addition to use in healthcare settings.
August 8, 2022:
 Association between AZD7442 (tixagevimab-cilgavimab) Administration and SARS-CoV-2 Infection, Hospitalization and Mortality. 7/29/22. Kertes J. Clin Infect Dis.
Association between AZD7442 (tixagevimab-cilgavimab) Administration and SARS-CoV-2 Infection, Hospitalization and Mortality. 7/29/22. Kertes J. Clin Infect Dis.
This retrospective, observational, “real world” study demonstrates lower rates of infection (3.5% vs. 7.2%), hospitalization (0.1 vs. 0.6%) and mortality (0 vs. 0.9%) when AZD7442 is administered pre-infection to a heterogenous group of highly immunosuppressed patients and compared to a similar group who chose not to receive the drug combination. The study coincided with an Omicron-dominated wave of COVID-19 in Israel (Dec. 2021- April 2022) and was designed to determine whether AZD7442, a human SARS-CoV-2 neutralizing monoclonal antibody combination, with the US trade name, Evusheld, was effective against the Omicron variant using a large Israeli healthcare system’s database. Among the immune suppressed patients contacted, 825 patients chose to participate and received AZD7442, while 4,299 declined and served as controls. Study results complement several ongoing and completed randomized controlled trials (PROVENT, TACKLE) and suggest the use of AZD7442 with its 90-day half-life for pre-exposure prophylaxis in individuals where immune-suppression is a concern.- Association of nirmatrelvir/ritonavir treatment on upper respiratory SARS-CoV-2 RT-PCR negative conversion rates among high-risk patients with COVID-19. 7/23/22. Li H. Clin Infect Dis.
Nirmaltrelvir/ritonavir (Paxlovid) given orally within 5 days of symptom onset to high-risk patients hospitalized with mild to moderate COVID-19, accelerated the RT-PCR conversion rate from positive to negative significantly. Median conversion time for a 258-patient cohort receiving the drug between March and April 2022 was day 10 (7-12 days) while a control cohort of 241 patients converted 7 days later on day 17 (12-21 days). Secondary outcomes included lingering PCR positivity on day 15 among untreated patients, resulting in a hazard ratio of 4.33 in favor of patients receiving the drug combination. Delayed negative conversion were also observed in patients with lower cycle threshold (Ct) during RT-PCR testing, suggesting the presence of greater viral loads. Although neither infectiousness nor diminishing viral loads were assessed directly, the accelerated RT-PCR conversion suggests reduced risk of viral shedding and disease transmission in patients receiving the antiviral.  Covid-19 Vaccination and the Timing of Surgery Following Covid-19 Infection. 7/15/22. Le S. Annals of Surgery.
Covid-19 Vaccination and the Timing of Surgery Following Covid-19 Infection. 7/15/22. Le S. Annals of Surgery.
Kaiser Permanente investigators reviewed 228,913 adult elective inpatient and outpatient surgeries, pre-pandemic and after vaccination availability, to assess whether vaccination status or type of anesthesia affected postoperative complication rates following SARS-CoV-2 infection. Postoperative emergency room visits and unscheduled hospitalizations were increased only for patients not fully vaccinated at the time of surgery, and if it occurred <30 days following a COVID diagnosis (n=373, adjusted HR 1.55). The increased risk was fully accounted for in those who had general anesthesia (adjusted HR 1.84). Risks were not elevated when surgery occurred more than 4 weeks following a COVID diagnosis. Authors noted “Current guidelines recommend deferring elective surgery for at least 7 weeks after Covid-19 diagnosis among patients who are asymptomatic at the time of surgery.” They recommend further study and liberalizing guidelines for those fully vaccinated or for whom general anesthesia can be avoided.
SAB Comment: This article prompted SAB review of a key cited reference that analyzed data from unvaccinated patients undergoing major elective surgery earlier in the pandemic. It contributed to guidelines suggesting a 7-8 week preoperative delay following a positive PCR. SARS-CoV-2 continues to evolve with multiple variants, the specter of Long COVID-19, and the concomitant evolution of vaccines and therapeutics. Consequently, decisions regarding timing of elective surgery following a COVID diagnosis are best individualized to each patient. We anticipate ongoing studies regarding the important question of how to assess and minimize perioperative risks following COVID. The Risk of Postoperative Complications After Major Elective Surgery in Active or Resolved COVID-19 in the United States. 2/22/22. Deng JZ. Annals of Surgery.
The Risk of Postoperative Complications After Major Elective Surgery in Active or Resolved COVID-19 in the United States. 2/22/22. Deng JZ. Annals of Surgery.
Using data from the COVID-19 Research Database, investigators studied the incidence of complications following 18 common major elective surgeries in unvaccinated patients who previously had PCR-confirmed SARS-CoV-2 infection (98.7% mild-moderate). Compared with 2,621 controls who underwent equivalent surgeries at least 30 days before a COVID diagnosis, those who had surgery within 4 weeks following a COVID diagnosis (n = 780) had substantially elevated adjusted odds ratio (aOR) for pneumonia (aOR 6.5), respiratory failure (aOR 3.4), pulmonary embolus (aOR 2.4) and sepsis (aOR 3.7). For those having surgery between 4 and 8 weeks after COVID (n = 445), only the aOR for pneumonia was higher at 2.4. For those having surgery more than 8 weeks following COVID (n=1633), no increased risk of complications was observed. Following a COVID diagnosis authors recommend waiting 8 weeks prior to major elective surgery, with due consideration for malignancies.- Dendrimer nanotherapy for severe COVID-19 attenuates inflammation and neurological injury markers and improves outcomes in a phase2a clinical trial. 7/20/22. Gusdon AM. Science Trans Med.
OP-101, a therapeutic nanoparticle with attached “tendrils” (a dendrimer), can place high concentrations of the anti-inflammatory/antioxidant N-acetyl cysteine (NAC) into activated macrophages and microglia. This multicenter, randomized, double-blind, phase 2a pilot study tested single-escalating doses (2mg/kg: n=6, 4mg/kg: n=6, or 8 mg/kg: n=5) vs placebo (n = 7) in severe COVID-19. All received SOC/corticosteroids. OP-101 at 4 mg/kg significantly decreased inflammatory markers; 4 and 8 mg/kg reduced neurological injury markers. Risk for mechanical ventilation/death at 30 and 60 days was 71% for placebo and 18% for pooled OP-101. At 60 days, 3/7 placebo-treated patients and 14/17 OP-101–patients survived without drug-related adverse events.
SAB Comment: Multiple ligands can be attached to injectable dendrimer nanoparticles to allow the novel targeting, treating, and even illuminating of specific inflammatory cells. In this first-in-human trial, macrophages and microglia, suspected culprits in COVID-19, were nanotherapy-targeted and “treated” with high concentration of NAC. The striking, potentially “game-changing” survival results reported here in severe, corticosteroid-treated COVID-19 requires confirmation in much larger multi-institutional studies. Neuropsychiatric exams should be included. - Effectiveness Associated With Vaccination After COVID-19 Recovery in Preventing Reinfection. 7/27/22. Lewis N. JAMA Netw Open.
In this cohort study of more than 95,000 Rhode Island residents from March 2020 to December 2021, including residents and employees of long-term congregate care (LTCC) facilities, completion of the primary vaccination series after recovery from COVID-19 was associated with 49% protection from reinfection among LTCC residents, 47% protection among LTCC employees, and 62% protection in the general population during periods when wild type, Alpha, and Delta strains of SARS-CoV-2 were predominant.
SAB Comment: The finding that in people who have recovered from COVID-19, subsequent completion of the primary vaccination series reduced the risk of reinfection by approximately half is shown here for the earlier variants, but that conclusion may not be extended to the Omicron variants without further study. - Efficacy and safety of intramuscular administration of tixagevimab-cilgavimab for early outpatient treatment of COVID-19 (TACKLE): a phase 3, randomised, double-blind, placebo-controlled trial. 6/10/22. Montgomery H. Lancet Respir Med.
This human SARS-CoV-2-neutralizing monoclonal antibody (NMA) combination, also referred to as AZD7442 or Evusheld, has an extended half-life of 90 days and is subject to an Astra Zeneca-funded, multicenter, international trial which began enrolling unvaccinated patients with seven days or less of mild COVID-19 symptoms and at least one major risk factor in January 2021. By July 2021, 910 patients (average age 46) had received either placebo (n = 454) or an intramuscular injection of 300 mg each of tixagevimab and cilgavimab (n = 456). Progression to severe illness or death from any cause through day 29 occurred in 18 patients receiving the NMA combination, compared to 36 who received placebo, resulting in a relative risk reduction of 50.5%.
In the pre-Omicron era, this NMA combination provided significant protection from severe disease without major side effects. As the trial is ongoing, future results may confirm in-vitro effectiveness to the Omicron variant.
SAB Comment: An associated editorial, titled “Early treatment to prevent progression of SARS-CoV-2 infection,” highlights the evolving role antivirals and monoclonal antibodies like Evusheld may play in the prevention and management of SARS-CoV-2 and its variants, and the protection of vulnerable populations from severe disease. - Efficacy of Antibodies and Antiviral Drugs against Omicron BA.2.12.1, BA.4, and BA.5 Subvariants. 7/20/22. N Engl J Med.
The efficacy of monoclonal antibodies against the circulating BA.2.12.1, BA.4 and BA.5 is unknown. Live virus neutralization laboratory testing showed the combination of casirivimab and imdevimab (REGEN-COV) inhibited all variants; however, neutralization was reduced vs. imdevimab alone. Casirivmab was inactive. The combination of tixagevimab and cilgavimab (Evusheld) was active vs all three variants as was cilgavimab. Tixagevimab only neutralized BA.2.12.1. Sotrovimab lost all inhibitory capability. Bebtelovimab neutralized all three and was the only monoclonal active at concentrations similar to the ancestral strain. Remdesivir, molnupiravir and nirmatrelvir (active component of Paxlovid) all neutralized variants at concentrations similar to ancestral stains.
SAB Comment: To date, there has been a lack of in vitro or clinical data to help decision making in selection of monoclonals and antiviral drugs to treat current circulating variants. These in vitro data may help a treating physician formulate a rationale for such decisions. - Long-term functioning status of COVID-19 survivors: a prospective observational evaluation of a cohort of patients surviving hospitalisation. 7/27/2022. Battistella LR. BMJ Open.
This paper from Brazil examines 801 patients hospitalized for at least 24 hours with SARS-CoV-2 from March-August 2020, who were recruited between October 2020 and April 2021 for evaluation of their long term functional status. Multiple formal evaluations of strength, mobility, and physical and mental function were administered, and help to quantify long COVID symptoms. In this group of patients, there was no difference in the functional status of those who needed invasive mechanical ventilation, and those who did not require it. The predominant findings were muscle weakness, reduced mobility, pain, anxiety, depression, breathlessness, insomnia and daytime sleepiness. Generalized fatigue was not present. - Symptoms and risk factors for long COVID in non-hospitalized adults. 7/25/2022. Subramanian A. Nat Med.
This is a study of symptoms from a UK primary care database during January 2020-April 2021. Non-hospitalized COVID-19 patients (486,000) were compared with propensity matched control patients who did not have a COVID-19 diagnosis with regard to the presence of 115 symptoms, from the day of a positive test until the end of the study. They report 42 symptoms associated with long COVID that are not among the 33 symptoms of long COVID which are designated by the World Health Organization. Symptoms with the largest hazard ratios were anosmia, hair loss, sneezing, ejaculation difficulty, and reduced libido. Risk factors for long COVID were female sex, ethnic minority, socioeconomic deprivation, smoking, obesity, and a wide range of comorbidities. Using symptom frequencies, the authors grouped long COVID into three phenotypes: one with a broad spectrum of symptoms, one with mainly respiratory symptoms, and one with mainly mental health and cognitive symptoms. The discussion is informative and spurs further investigation.
August 3, 2022:
- Antibody evasion by SARS-CoV-2 Omicron subvariants BA.2.12.1, BA.4, & BA.5. 7/5/22. Wang Q. Nature.
The authors performed systematic antigenic analyses of Omicron subvariants finding BA.2.12.1 only modestly (1.8-fold) more resistant to sera from vaccinated and boosted individuals than BA.2. However, BA.4/5 is substantially (4.2-fold) more resistant and thus more likely to lead to vaccine breakthrough infections. Among therapeutic antibodies authorized for clinical use, only bebtelovimab retains full potency against both BA.2.12.1 and BA.4/5. Serum neutralization assays used sera from persons who received three shots of mRNA vaccines, others who received mRNA vaccines before or after non-Omicron infection, vaccinated patients with BA.1 or BA.2 breakthrough infection as well as pseudoviruses containing point mutations. The Omicron SARS-CoV-2 lineage continues to evolve, successively yielding subvariants that are both more transmissible and more evasive to antibodies.
SAB Comment: Bebtelovimab (Eli Lilly) has been authorized under EUA since 2/22 for mild-to-moderate COVID-19 in patients older than 12 years and weighing at least 40 kg who are at high risk for progression to severe disease. It is given as a single IV injection, within 7 days of symptom onset. Readers may access current NIH Therapeutic Guidelines, including use of bebtelovimab, on the IARS COVID-19 Published Guidelines and Reviews web page.  Extended prone positioning duration for COVID-19-related ARDS: benefits and detriments. 7/8/22. Walter T. Crit Care.
Extended prone positioning duration for COVID-19-related ARDS: benefits and detriments. 7/8/22. Walter T. Crit Care.
This retrospective observational study in France from March 2020-April 2021 evaluated 81 COVID-19 patients, using a strategy, originally implemented for organizational and human resources purposes. It was based on extending the duration of proning sessions (PP) up to 48 hours in patients ventilated for COVID-19-related ARDS. The primary objective, the occurrence of pressure injury, was observed in 26% of patients and was equivalent to patients who had PP sessions of shorter duration for non-COVID-19-related ARDS. The presence of skin injury, the most common complication of PP, correlated with cumulative duration of PP sessions and length of ICU stay, not the duration of each session. Extended PP significantly reduced staff viral exposure and workload, allowing for most position changes during the daytime. Longer proning sessions were also associated with continued improvements in ventilatory parameters over the extended time.- The COVID-19 pandemic and its consequences for chronic pain: a narrative review. 7/18/22. Shanthanna H. Anaesthesia.
This is a narrative review of 3,859 published reports. It succinctly reviews new onset chronic pain after SARS-CoV-2 infection, the influence of the pandemic on patients with pre-existing chronic pain, possible pain mechanisms associated with SARS-CoV-2, and treatments being considered to address post-COVID-19 pain. Both new onset post-COVID-19 pain and ongoing chronic pain increased. Risk factors were social isolation, lack of psychological support, female sex, lower level of education, reduced physical activity and disabled employment status. Tables summarize key findings on COVID-19-associated pain and headache, and the effect of the pandemic on chronic pain patients and the use of steroids for pain interventions. A diagram illustrates possible mechanisms of pain and associated symptoms with COVID-19. Finally, vaccination and the use of steroids and opioids are discussed. - Tocilizumab Versus Baricitinib in Patients Hospitalized With COVID-19 Pneumonia and Hypoxemia: A Multicenter Retrospective Cohort Study. 7/5/2022. Roddy J. Crit Care Explor.
This study’s real-world impetus arose when IL-6 inhibitor Tocilizumab became unavailable to a Wisconsin health care system in August 2021 and Baricitinib was used in its place. A retrospective study of 382 hospitalized patients with SARS CoV-2 pneumonia requiring supplemental oxygen showed no difference in primary outcome, defined as hospital discharge alive and free of mechanical ventilation within 60 days.
July 18, 2022:
 Maternal Vaccination and Risk of Hospitalization for Covid-19 among Infants. 6/22/22. Halasa NB. N Engl J Med.
Maternal Vaccination and Risk of Hospitalization for Covid-19 among Infants. 6/22/22. Halasa NB. N Engl J Med.
This multicenter, case-control, test-negative study assessed the effectiveness of maternal 2-dose mRNA COVID-19 vaccination during pregnancy against hospitalization for COVID-19 disease among infants younger than 6 months. Protection was 52% overall (80% during the Delta period, and 38% during the Omicron period). Of 537 case infants hospitalized for COVID-19, only 16% were born to mothers fully vaccinated during pregnancy vs. 29% of 512 hospitalized controls. Among case infants, 21% were admitted to ICU; 12% received mechanical ventilation or vasoactive infusions. Two infants died from COVID-19 and 2 received ECMO treatment; none of these 4 infants’ mothers had been vaccinated during pregnancy. Effectiveness was greater if vaccination occurred after 20 weeks of gestational age than earlier in pregnancy (69% vs. 38%). However, due to well-documented risks of COVID-19 during pregnancy, this did not lead to a recommendation to delay vaccination. The results are discussed in a well-written accompanying editorial.
July 11, 2022:
- American Society of Hematology living guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID-19: March 2022 update on the use of anticoagulation in critically ill patients. 6/24/2022. Cuker A. Blood Adv.
These evidence-based guidelines of the American Society of Hematology are an update from the prior guidelines from February 2021 examining the use of anticoagulation in critically ill patients with COVID-19. The panel issued a recommendation in favor of prophylactic-intensity over therapeutic-intensity anticoagulation in critically ill patients with COVID-19-related acute illness who do not have suspected or confirmed VTE. This manuscript helps clinicians taking care of COVID-19 with the most up to date information. - Benefits of plasma exchange on mortality in patients with COVID-19: a systematic review and meta-analysis. 6/16/2022. Qin J. Int J Infect Dis.
Controlled clinical trials on the use of therapeutic plasma exchange (TPE) for hospitalized COVID-19 patients, compared with standard of care (SOC), were reviewed. Six trials, enrolling 173 participants who received TPE and 170 controls, met inclusion criteria. Only one study involving 87 participants was randomized. Mortality following TPE was significantly lower than SOC – ~18% vs. ~45% (RR 0.41, 95% CI 0.24-0.69; P = 0.0008). Authors postulate that TPE may interrupt the progression of cytokine storm syndrome in critically ill patients by removing increased cytokines and inflammatory mediators, and call for larger randomized trials. Meanwhile, TPE should be considered for severely ill COVID-19 patients. - Hospitalization and Emergency Department Encounters for COVID-19 After Paxlovid Treatment – California, December 2021-May 2022. 6/23/22. Malden DE. MMWR Morb Mortal Wkly Rep.
To assess hospital admissions and emergency care visits after a course of Paxlovid using the Kaiser Permanente Health Care System’s database, the authors found that among 5,200 patients with mild to moderate disease, advanced age or concomitant medical conditions, only 6 patients (0.11%) treated with Paxlovid were identified with COVID-19-related hospitalization and 39 (0.74 %) had ED encounters 5-15 days after treatment was dispensed.
The likely mechanisms causing the recurrence of COVID-19 symptoms after early treatment with Paxlovid are transient suppression of viral replication before natural immunity is sufficient to complete viral clearance. This could not be proven since viral sequencing was not done and recovery from the initial infection was not assessed. Less likely and also not proven in this study are viral reinfection or emergence of treatment-resistant mutations. - Long COVID symptoms in SARS-CoV-2-positive children aged 0-14 years and matched controls in Denmark (LongCOVIDKidsDK): a national, cross-sectional study. 6/25/2022. Kikkenborg Berg S. Lancet Child Adolesc Health.
Denmark’s COVID national database contained 38,152 children 0-14 years old (who tested positive between 1/2020 and 7/2021), who were matched with 4 controls of the same age and sex from the civil registration system. Mothers of all were invited to participate in a survey in July 2021 of 23 of the most common long COVID symptoms, the Pediatric Quality of Life Inventory (PedsQL) and the Childrens Somatic Symptoms Inventory-24 (CSSI-24). Data was analyzed according to age groups and duration of symptoms. Cases were more likely to report symptoms lasting more than 2 and 3 months than controls. Children older than 4 years in the control group had lower PedsQL scores than the cases, and both groups had scores lower than the norm for healthy children. The cases represented 4.2% of children in Denmark; by comparison, 58% tested positive between 12/15/2021 and 2/15/2022. - Oral Sabizabulin for High-Risk, Hospitalized Adults with Covid-19: Interim Analysis. 7/6/22. Barnette KG. NEJM Evidence.
Sabizabulin is a novel, oral, microtubule disruptor with antiviral and anti-inflammatory activities. Investigators studied sabizabulin for up to 21 days in a randomized (2:1 vs. placebo), multinational phase 3 trial, in hospitalized patients with moderate to severe COVID-19 at high risk of ARDS and death. After 204 patients were assigned, the trial was stopped for efficacy when a planned interim analysis of 150 patients revealed 60-day mortality (primary end point) was 20.2% (19 of 94) for sabizabulin vs. 45.1% (23 of 51) for placebo, an absolute reduction of 25%, and a 55% relative mortality reduction. Reduced mortality was seen regardless of concomitant treatments such as prednisone and remdesivir, baseline WHO ordinal score, sex, age, comorbidities, or geographic location (US or mostly Brazil). Secondary end points including days of hospitalization, ICU, and mechanical ventilation all strongly and significantly favored sabizabulin, as did serious adverse events.
SAB Comment: This study, conducted from May 18, 2021 to January 31, 2022, and sponsored by sabizabulin’s developer, Veru Inc., showed a striking 55% relative mortality reduction vs. placebo. Colchicine, also a microtubule inhibitor, failed in clinical trials. Sabizabulin is touted to have unique physiochemical properties. Of note, the placebo mortality rate is higher than other recent severe COVID studies. The small study size begs for larger independent studies to confirm potentially exciting, practice-changing results. Veru has applied to the FDA for an Emergency Use Authorization. - Viral dynamics of Omicron and Delta SARS-CoV-2 variants with implications for timing of release from isolation: a longitudinal cohort study. 7/23/22. Bouton TC. Clin Infect Dis.
Starting January 2022, CDC guidelines recommended isolation for 5 days from symptom onset, or a positive test if asymptomatic, followed by 5 days of masking. This university campus, longitudinal study (n=92 subjects, mean age 22, 17 Delta, 75 Omicron) examined 10 consecutive days of PCR and antigen tests vs culture positivity. Beyond day 5, 17% remained culture positive (latest day 12) and no difference in time to culture conversion by variant or vaccination status. Most culture-converted by day 6. Conclusion: antigen testing may provide reassurance of lack of infectiousness, though masking days 6-10 is supported by 17% that remained culture positive after day 5.
SAB Comment: In this study, culture positivity was a surrogate for infectiousness, which is a reasonable assumption, but never confirmed. However, it does suggest that in an otherwise “healthy” group in their early twenties (e.g., college students, medical students) a negative antigen test is reassuring irrespective of strain, but mask-wearing remains advisable until day 10 post-symptom onset. This information on the value of antigen tests should not necessarily be extrapolated to other cohorts including older individuals, immunocompromised, and those many months out from primary vaccination or boosters. - When to test for COVID-19 using RT-PCR: a systematic review. 6/27/2022. Dos Santos PG. Int J Infect Dis.
This review of 90 studies from 31 countries involving 6,831 patients corroborates current practice. It is estimated that the mean incubation time of the virus is 5 days and that patients generally become positive 3 days after symptom onset and negative approximately 15 days after that. The three most frequent symptoms of COVID-19 are fever, cough, and dyspnea. The best time to perform RT-PCR is between the 1-7 day of symptom onset, with the highest percentage of positive tests on the 7th day, however some studies showed a mean time for first positive PCR at 14-16 days.
June 27, 2022:
- ASA and APSF Statement on Perioperative Testing for the COVID-19 Virus. 6/16/22. ASA & APSF.
SAB Comment: Readers are encouraged to review the full publication. A few key points, including the potential use of community transmission metrics to guide the use of universal testing, are summarized below:
SCREENING: All patients should be screened for COVID-19 symptoms and for close contact to someone diagnosed with COVID-19 in the past 10 days.
TESTING: The use and limitations of PCR for SARS-CoV-2 testing are reviewed. If a patient tests positive, elective surgical procedures should be delayed. Antibody testing is not recommended for perioperative screening.
COMMUNITY TRANSMISSION METRICS: Two metrics, based on cases/100K in the last 7 days and % test positivity in the last 7 days, are available by community on the CDC website. When levels are graded substantial to high, universal use of PCR testing is recommended. If community transmission is low to moderate, the patient is asymptomatic, up-to-date in vaccination and having a lower-risk procedure, facilities could consider a more permissive approach to perioperative testing.
A number of specific situations are reviewed, including cases of immunosuppressed or other patients likely to carry transmissible virus for longer than 10 days after infection. - BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection. 6/17/2022. Cao Y. Nature.
This lab study from China characterizes the evolution of newer Omicron variants, and how those variants escape antibody neutralization from prior SARS-CoV-2 infections, vaccinations and antibody products. Focusing on the original Omicron variant (BA.1) as a comparator, the authors explain the various genetic changes in the spike protein in subsequent Omicron variants (BA.2, BA.2.12.1, BA.2.13, BA.4 and BA.5) and measure the neutralization capacity of sera after vaccination, infection as well as that of therapeutic antibody products. Their results indicate that Omicron may evolve mutations to evade the humoral immunity elicited by BA.1 infection, suggesting that BA.1-derived vaccine boosters may not achieve broad-spectrum protection against new Omicron variants.
SAB Comment: This detailed and intricate paper explains the possible genetic mechanisms of the clinically known behavior of evolving Omicron variants. - Effectiveness of Homologous and Heterologous Covid-19 Boosters against Omicron. 5/25/2022. Accorsi EK. N Engl J Med.
In this Research Letter, authors from the CDC report on a test-negative, case–control analysis to assess the effectiveness of four vaccination regimens against symptomatic SARS-CoV-2 infection during omicron predominance. Data looked at positivity rates from 512,928 rapid and laboratory-based nucleic acid amplification tests at 7046 testing centers across the US. All regimens that included a booster dose offered protection against symptomatic omicron infection compared with no vaccination. Vaccine effectiveness was highest for regimens that included a booster dose of an mRNA vaccine and was lowest for the homologous J&J/J&J regimen. A single mRNA booster dose in persons who received primary J&J vaccination provided protection close to three-dose mRNA vaccination. Data support the current recommendation of an mRNA vaccine booster at least 2 months after single-dose primary J&J vaccination or at least 4 months after a J&J booster dose. - Effects of Previous Infection and Vaccination on Symptomatic Omicron Infections. 6/15/2022. Altarawneh HN. N Engl J Med.
This matched, test negative, case-controlled study in Qatar, from 12/21-2/22, looked at the effectiveness of previous infection and vaccination for prevention of symptomatic omicron (BA.1, BA.2) infection or severe disease. Data were obtained from the integrated nationwide digital health information program. Cases consisted of all symptomatic individuals with positive PCR tests; matched controls had no positive PCR on their chart. The odds of previous infection or vaccination among cases and controls determined effectiveness. Previous infection, 3 doses of mRNA vaccine, or previous infection and 2 doses of mRNA vaccine were roughly 50% effective against symptomatic disease. Two vaccine doses alone were ineffective, and 3 doses and previous infection was more than 70% effective. All were more than 70% effective against severe disease, with 3 doses of vaccine, or previous infection with 2 or 3 doses of vaccine conferring greater than 95% effectiveness. - Efficacy and safety of intramuscular administration of tixagevimab-cilgavimab for early outpatient treatment of COVID-19 (TACKLE): a phase 3, randomised, double-blind, placebo-controlled trial. 6/10/2022. Montgomery H. Lancet Respir Med.
This human SARS-CoV-2-neutralizing monoclonal antibody (NMA) combination, also referred to as AZD7442, with an extended half-life of 90 days is subject to an Astra-Zeneca funded, multicenter, international trial which began enrolling unvaccinated patients with mild COVID-19 symptoms and at least one major risk factor in January 2021. By July 2021, 910 patients (average age 46) had received either placebo (n=454) or an intramuscular injection of 300 mg each of tixagevimab and cilgavimab (n=456). Progression to severe illness or death from any cause through day 29 occurred in 18 patients receiving the NMA combination, compared to 36 who received placebo, resulting in a relative risk reduction of 50.5%.
In the pre-omicron era, this NMA combination provided significant protection from severe disease without major side effects. As the trial is ongoing, future results may confirm in-vitro effectiveness to the omicron variant. - Evolving outcomes of extracorporeal membrane oxygenation during the first 2 years of the COVID-19 pandemic: a systematic review and meta-analysis. 5/23/22. Ling RR. Crit Care.
This article reviews the extracorporeal membrane oxygenation (ECMO) experience worldwide using a meta-analysis and systematic review. Of 4,522 citations, they included 52 studies comprising 18,211 patients. The pooled mortality rate among patients with COVID-19 requiring ECMO was 48.8%. Mortality was higher among studies which enrolled patients later in the pandemic as opposed to earlier (1st half 2020: 41.2%, 2nd half 2020: 46.4%, 1st half 2021: 62.0%, 2nd half 2021: 46.5%, p value = 0.0014). Predictors of increased mortality included age, an increased proportion of patients receiving corticosteroids, and a shorter duration of ECMO run. These data help characterize changes in COVID-19 with respect to outcome using ECMO. - Immune boosting by B.1.1.529 (Omicron) depends on previous SARS-CoV-2 exposure. 6/14/22. Reynolds CJ. Science.
Even in those triple vaccinated, Omicron (B.1.1.529) can evade immune defenses. These investigators studied triple mRNA-vaccinated healthcare workers (TVHCW) with diverse COVID histories. Before suffering Omicron infections, TVHCW showed robust in vitro blood antibody, B- and T-cell immunity to all variants of concern (VOC) EXCEPT Omicron. Prior Alpha infections singularly dampened Omicron response duration. Previously infection-naive TVHCW who then suffered Omicron infections showed boosted immune responses to all VOC EXCEPT Omicron. TVHCW with a history of prior COVID infection followed by Omicron infections had negligible immune boosting. Interestingly, a prior Wuhan Hu-1 infection abrogated Omicron immune defenses. Inability of Omicron to boost itself invites reinfection.
SAB Comment: It had been assumed that SARS CoV-2 infections would boost immune defenses against SARS-CoV-2, and that “hybrid immunity” (both vaccine and infection) would provide an even greater boost. This study shows that Omicron fails to boost immune responses in TVHCWs (remember-vaccine is Wuhan strain-based). The failure was especially profound if subjects had past Wuhan or Alpha infections termed, “hybrid immune-dampening.”
This study predicts: 1) Omicron re-infection is possible/probable, and 2) vaccination + the specific SARS CoV-2 strain (e.g., Wuhan, Alpha, Delta) infection history will shape (“imprint”) the strength and durability of responses.
Omicron produces poor antibody, T- and B-cell immunogenicity against itself, and predicts vaccines based purely upon Omicron-sequences may produce poor immunogenicity and protection unless paired with ancestral-sequence-based vaccine. - Impact of dexamethasone on the incidence of ventilator-associated pneumonia in mechanically ventilated COVID-19 patients: a propensity-matched cohort study. 6/13/2022. Scaravilli V. Crit Care.
Authors compare the ventilator acquired pneumonia (VAP) rate of 178 patients early in the Covid-19 epidemic given Dexamethasone 6 mg/day intravenous for 10 days from hospital admission to the same rate in 178 propensity matched patients who did not receive Dexamethasone. 56% of the Dexamethasone treated patients developed a VAP versus 34% of those not treated (RR 1.61, p = 0.0001) after similar time from hospitalization, ICU admission and intubation. VAPs were similarly due to G+ bacteria (mostly Staphylococcus aureus) and G− bacteria (mostly Enterobacterales). VAP was associated with almost doubled ICU and hospital LOS and invasive mechanical ventilation, and increased mortality (RR 1.64) with no differences between these patient groups. - N95 respirators: quantitative fit test pass rates and usability and comfort assessment by health care workers. 5/29/22. Ng I. Medical Journal of Australia.
Healthcare workers (n = 2,161) at the Royal Melbourne Hospital underwent quantitative fit testing of N95 respirators per Australian Infection Control Expert Group protocol, each with at least three of four types: semi-rigid cup, flat-fold cup, duckbill, and three-panel flat-fold. Images are available here. The overall fit test pass rates were 65% for the semi-rigid cup respirators, 32% for the flat-fold respirator, 59% for the duckbill respirators, and 96% for the three-panel flat-fold respirator. Three hundred seventy-eight participants completed a detailed comfort and usability survey. Overall comfort and assessment ratings differed significantly by type. Median overall comfort and overall assessment values were highest for the three-panel flat-fold model and lowest for the semi-rigid cup model. These results may inform institutional procurement decision-makers as well as individuals who may not have access to quantitative testing to enhance respiratory protection. - Nasal Spray of Neutralizing Monoclonal Antibody 35B5 Confers Potential Prophylaxis Against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Variants of Concern (VOCs): A Small-scale Clinical Trial. 6/6/22. Lin Y. Clin Infect Dis.
These investigators studied a nasal spray formulation of a SARS-CoV-2 neutralizing IgG1 monoclonal antibody (“35B5”). In 30 healthy, disease-naïve but vaccinated volunteers, nasal mucosal samples were assayed against spike proteins of the wild type (WT) and variants of concern (VOC) including Omicrib (B.1.1.529) at 0h and again at 12h-72h post-spray. Samples collected within 24 hours following a single spray neutralized WT and all VOC. Protection efficacy dropped to 60% and 20% at 48h and 72h, respectively. At a time where most monoclonals have lost efficacy especially against Omicron, the 35B5 nasal spray formulation may enhance SARS-CoV-2 prevention especially in unvaccinated and high-risk populations.
SAB Comment: This novel means of prevention at upper airway sites of viral introduction will need assessment in large-scale clinical trials. However, if proven effective, easy-to-administer intranasal monoclonals against VOC including Omicron would fill an unmet clinical need for those anticipating potential exposure including medical settings. - Neutralization Escape by SARS-CoV-2 Omicron Subvariants BA.2.12.1, BA.4, and BA.5. 6/22/22. Hachmann N. NEJM.
These authors evaluated the neutralizing antibody titer levels against the reference WA1/2020 (ancestral) isolate of SARS-CoV-2 along with Omicron subvariants BA.1, BA.2, BA.2.12.1, and BA.4 or BA.5 in 27 participants who had been vaccinated and boosted with messenger RNA vaccine BNT162b2 (Pfizer–BioNTech). As expected, the neutralizing antibody titer was lower by a factor of 6 to 21 against the Omicron variants compared with the response against the WA1/2020 isolate. As compared with the median neutralizing antibody titer against the BA.1 subvariant, the median titer against the other omicron subvariants was lower by a factor of 2.2 against the BA.2.12.1 subvariant and by a factor of 3.3 against the BA.4 or BA.5 subvariant. The authors also tested neutralizing antibodies in an additional 27 participants (only one of whom had ever been vaccinated) who had been infected with the BA.1 or BA.2 subvariant a median of 29 days earlier. As compared with the median titers against the BA.1 subvariant, the median titer was lower by a factor of 1.5 against the BA.2.12.1 subvariant and by a factor of 2.9 against the BA.4 or BA.5 subvariant. - Rapid, scalable assessment of SARS-CoV-2 cellular immunity by whole-blood PCR. 6/13/22. Schwarz M. Nature Biotechnology.
Low T-cell activation measurements to SARS-CoV-2 is predictive of COVID-19 breakthrough and need for revaccination. T-cell assays are difficult and rarely performed. These investigators developed fast, high-throughput quantitative PCR assays for T-cell activation. The tests stimulate whole-blood cells with SARS-CoV-2 antigens. Viral-specific T-cells secrete IFN-γ, which then stimulate monocytes to produce the cytokine CXCL10 mRNA which correlates and proved a proxy for SARS COV-2 antigen-specific T-cells activation. These assays may allow large-scale monitoring of both the magnitude and duration of functional T-cell immunity to SARS-CoV-2. In vulnerable populations, such screening may predict breakthrough infections and help prioritize revaccination strategies. - Risk of long COVID associated with delta versus omicron variants of SARS-CoV-2. 6/18/2022. Antonelli M. Lancet.
SAB Comment: Although many authors do not consider a 28-day period of post-infection symptoms sufficient to be termed “long COVID” the information in this article may represent early data regarding the probability of longer symptom persistence.
To determine the relative risk of persistent symptoms, this UK case-control observational study compared 56,000 previously vaccinated adults who tested positive for COVID-19 and logged symptom data via an app for at least 28 days during the Omicron wave with over 40,000 previously vaccinated, matched controls infected during the Delta wave. Overall, persistent or new self-reported symptoms at 28 days post diagnosis were less than half as likely after Omicron (4.5% vs. 10.8%). Adjusted odds ratios ranged from 0.26 for those who were vaccinated over 6 months previously to 0.50 for those vaccinated less than 3 months previously. There was little variation between those under or over 60 years of age. - Short-term, relative effectiveness of four doses versus three doses of BNT162b2 vaccine in people aged 60 years and older in Israel: retrospective, test negative, case-control study. 5/24/22. Gazit S. BMJ.
Patients older than 60 years from a single Israeli healthcare provider were studied during Omicron dominance to determine the relative effectiveness of 4 vs. 3 Pfizer vaccine doses against a +PCR, and against COVID-related hospitalization or death. Approximately twenty-eight thousand people received the 4th dose and were compared to ~78,000 who were eligible but had not. A 4th dose provided peak relative protection from infection of 65% at week 3; however, by week 10 it was only 22% greater than 3 doses. Relative protection from severe disease or death, both occurring in <1% of subjects, was more durable in the 4th dose group, remaining >72% greater throughout the 10-week study period. - The role of extracorporeal membrane oxygenation in COVID-19. 6/6/2022. Dalia AA. J Cardiothorac Vasc Anesth.
This article reviews the ECMO protocol at Mass General Hospital for COVID-19 patients. This well-written paper details indications, protocols for laboratory evaluation before and during ECMO, cannulation protocols, weaning protocols, and how to protect providers and other non-COVID-19 patients. The protocols are well thought out and based upon experience. It is a useful reference for those involved with ECMO for the COVID-19 patients and those evaluating patients for ECMO.
June 13, 2022:
- Baricitinib versus dexamethasone for adults hospitalised with COVID-19 (ACTT-4): a randomised, double-blind, double placebo-controlled trial. 5/26/22. Wolfe CR. Lancet Respir Med.
This trial aimed to compare the effectiveness of 2 immunomodulators, baricitinib and dexamethasone, given orally in combination with remdesivir, in preventing progression to mechanical ventilation or death in hospitalized patients with COVID-19. Between December 2020 and April 2021, it enrolled 1010 patients whose oxygen requirements ranged from supplemental to noninvasive mechanical ventilation at 67 sites. While there was no difference in mechanical ventilation-free survival by day 29 between these groups (odds ratio 1.01), treatment-related adverse events and adverse events in general, some life threatening, among patients receiving dexamethasone exceeded those receiving baricitinib significantly. The number needed to harm for one additional severe or life threatening adverse event with dexamethasone was 12·5, suggesting that immunomodulation should be tailored according to individual patient’s risk profile and other variables. - Effectiveness of Paxlovid in Reducing Severe COVID-19 and Mortality in High Risk Patients. 6/2/22. Najjar-Debbiny R. Clin Infect Dis.
This retrospective cohort study examines the effectiveness of Paxlovid on COVID-19 progression and mortality in a real life, uncontrolled setting, during the early Omicron phase using an Israeli data base of 180,000. Among 4737 patients receiving Paxlovid within 5 days of a positive PCR test and having at least one risk factor, a significant decrease in progression to severe COVID-19 and mortality occurred resulting in an HR of 0.54%, compared to full vaccination with an even more favorable outcome and an HR of 0.20. Paxlovid appears to be more effective in older and immunosuppressed patients, and patients with underlying neurological or cardiovascular disease. Vaccination remained the most effective means of preventing progression of the disease. Paxlovid had received an emergency release by the FDA based on pre-Omicron data obtained following the EPIC-HR controlled trial. This study proves its effectiveness in the Omicron era. - Limited cross-variant immunity from SARS-CoV-2 Omicron without vaccination. 5/18/22. Suryawanshi RK. Nature.
This complex study applies basic laboratory research to answer the question whether widespread infections with the Omicron variant will eventually lead to herd immunity and end the COVID-19 epidemic. After creating mouse models susceptible to various SARS CoV-2 variants (WA1, Delta and Omicron), the authors compared their impact on respiratory tract pathology and immune markers and showed that Omicron infections were less severe and resulted in a diminished inflammatory response. When collecting convalescent serum from mice and humans, a diminished humoral immune response to Omicron infections compared to other variants indicated limited cross-variant neutralization induced by Omicron in mice and humans. Sera collected from vaccinated individuals experiencing breakthrough infections with Omicron developed higher neutralization titers against all variants, demonstrating that Omicron enhances pre-existing immunity but lacks protection against non-Omicron variants in the unvaccinated. Sera collected from vaccinated individuals experiencing breakthrough infections with Omicron developed higher neutralization titers against all variants, demonstrating that Omicron enhances pre-existing immunity but lacks protection against non-Omicron variants in the unvaccinated. - Long COVID after breakthrough SARS-CoV-2 infection. 5/25/2022. Al-Aly Z. Nat Med.
This analysis from the US VHA database during January- October 2021 sought to characterize long COVID in 33,940 vaccinated people experiencing breakthrough infection (BTI). Compared to 4,983,491 contemporary controls, the HR of death after the acute phase of BTI (between 30 days and 6 months) was 1.75, The HR of at least one post-acute sequelae was 1.5. When compared to 113,474 people with SARS-CoV-2 who were not previously vaccinated, the HR of death after the acute phase of BTI was 0.66 and of post acute sequelae was 0.85. BTI also resulted in increased death and post-acute sequelae compared to seasonal influenza. The results are also reported throughout as an excess burden per 1000 people. The data include comparisons regarding acute phase treatment, vaccine type, and immune compromised status. The authors conclude that vaccination confers only partial protection in the post-acute phase of the disease and should not be the sole strategy for mitigating the long term health consequences of SARS-CoV-2 infection. - Protection and Waning of Natural and Hybrid Immunity to SARS-CoV-2. 5/25/2022. Goldberg Y. N Engl J Med.
This statistically intricate study from Israel assessed the level of protection afforded by SARS-CoV-2 infection (natural immunity) compared with vaccine induced immunity, and hybrid immunity (natural immunity plus various vaccine doses). The study followed PCR positivity in the various immunity cohorts during the Israeli Delta surge in August to October, 2021 at a time when the population was receiving the BNT162b2 (Pfizer) vaccine. Immunity waned in all cohorts, with a steady decrease in protection over time. In the recovered, unvaccinated cohort, the rates of confirmed infection were similar to those with hybrid immunity. The adjusted rates of confirmed infection among the recovered, unvaccinated subcohorts were lower than those among the two-dose subcohorts when the time since the last immunity-conferring event was similar; nevertheless, the protection in the two-dose cohort could be restored by the administration of a booster shot. - Safety and immunogenicity of a live-attenuated influenza virus vector-based intranasal SARS-CoV-2 vaccine in adults: randomised, double-blind, placebo-controlled, phase 1 and 2 trials. 6/1/22. Zhu F. Lancet Respir Med.
In this randomized, single-center, double-blind, placebo-controlled trial in healthy COVID-naïve adults, these investigators published the first intranasal vaccine results in humans. The Phase 1 (n=63 subjects), phase 2 (n=724) and phase 2-extension (n=297) trial arms administered a live-attenuated influenza virus vector-based SARS-CoV-2 spray (“dNS1-RBD”), 2 doses, 14 days apart. Peripheral blood showed both weak cellular (40-46% conversion) and IgG and IgA humoral responses (10-13% conversion). Secretory IgA (nasopharynx) conversions and concentrations were also weak. “dNS1-RBD” was well tolerated and without serious adverse effects (0-12 months). Despite apparent “weak” effects, this vaccine may be effective in a real-world, phase-3 trial, which is ongoing. - Safety of COVID-19 Vaccination in US Children Ages 5-11 Years. 5/18/2022. Hause AM. Pediatrics.
The authors analyzed data from 3 US safety monitoring systems and found a low incidence of adverse events and rates of postvaccination myocarditis that were substantially lower than in older children. The systems reviewed included v-safe, a voluntary smartphone-based system that monitors reactions and health effects; the CDC and FDA’s Vaccine Adverse Events Reporting System (VAERS), and the Vaccine Safety Datalink (VSD), an active surveillance system that monitors electronic health records for prespecified events, including myocarditis. Among 48,795 children ages 5–11 years enrolled in v-safe, most reported reactions were mild-to-moderate. VAERS received 7,578 adverse event reports; 97% were non-serious. On review of 194 serious VAERS reports, 15 myocarditis cases were verified (reporting rate 2.2 per million doses). In VSD, no safety signals were found. - Virological characteristics of the SARS-CoV-2 Omicron BA.2 spike. 5/14/22. Yamasoba D. Cell.
Authors review the mechanism of infection of all SARS-CoV-2 variants and studied infectivity and immune escape of BA.2, which has over 30 mutations compared with the Wuhan strain. Data suggest that similar to BA.1, BA.2 is highly resistant to antisera induced by vaccination or infection with other SARS-CoV-2 variants as well as three therapeutic antiviral antibodies. BA.2 has a 1.4-fold higher effective reproductive rate than BA.1 as well as higher fusion potential and pathogenic potential. BA.2 was 4-fold more resistant to BA.1-infected sera from convalescents without full vaccination. Multiple studies using convalescent human, hamster, and murine sera demonstrated that BA.1-induced humoral immunity is less effective against BA.2, but not vise versa. Virologic features and proposed mechanistic consequences are reviewed.
May 27, 2022:
- Health outcomes in people 2 years after surviving hospitalisation with COVID-19: a longitudinal cohort study. 5/14/22. Huang L. Lancet Respir Med.
Outcome of 1,192 patients 6 months, 12 months, and 2 years posthospitalization for COVID-19 between 1/7/20 and 5/29/20 in Wuhan, China is the topic of this longitudinal observational study. In addition, subjects were matched 1:1 by age, sex, and comorbidities, to a data set, created at the 12-month stage of 3,383 community-dwelling adults without previous SARS-CoV-2 infection. A subset of approximately 350 patients received pulmonary function studies and high resolution chest CT scans, with further CT scans only for those with abnormal lung images. All survivors underwent physical exam, routine labs, six-minute walk test and multiple standard questionnaires. Highlights of multiple results at 2 years: 55% had at least one symptom, 14% had dyspnea by the modified British Medical Research Council scale and 12% had anxiety/depression by the Health Related Quality of Life assessment. In addition, 89% had returned to their original work. Compared to controls, those who had respiratory support during hospitalization had reduced diffusion capacity, reduced residual volume, and reduced total lung capacity. The authors conclude further study is needed regarding the possibility of emerging pulmonary fibrosis. - Surgical Triage and Timing for Patients with COVID: A Guidance Statement from the Society of Thoracic Surgeons. 5/20/22. Grant MC. Ann Thorac Surg.
In this statement, universal preoperative testing for SARS-CoV-2, preferably PCR, is recommended. Further guidance, to be individualized, is dictated by a combination of procedure urgency, COVID-19 illness severity, and the present tier of the institution’s COVID-19 response, as outlined in the full publication. Example: For asymptomatic patients screening positive for SARS-CoV-2, it is recommended to defer non-urgent cardiac procedures at approximately 4-8 weeks. Patients with a procedural delay greater than 90 days from a positive test result should undergo repeat preoperative COVID-19 testing to screen for potential reinfection; prior testing following infection increases false positives, particularly with PCR. There is no convincing evidence that the type of anesthetic, airway management, or the use of regional anesthesia is associated with more favorable postoperative outcomes following recent COVID-19. - The Influence of the COVID-19 Pandemic on Intensivists’ Well-Being: A Qualitative Study. 5/14/2022. Vranas KC. Chest.
These investigators designed a semi-structured paid interview for 33 intensivists from 6 states to gauge the impact of the pandemic on their well-being. Through an elaborate method called “Inductive thematic analysis,” they reported that intensivists, due to visitor restrictions, experienced moral distress and helplessness in their day-to-day practice in the ICU leading to a variety of ailments including exhaustion, burnout, and PTSD. The perceived lack of support from colleagues and hospital hierarchy had negative impacts on patients, families and staff. The suggested mitigation measures include proactive provision of mental health resources, back-up schedules and recognition by their institutions to avoid future ramifications and decline of the workforce. - Thromboembolic prevention and anticoagulant therapy during the COVID-19 pandemic: updated clinical guidance from the anticoagulation forum. 5/17/2022. Barnes GD. J Thromb Thrombolysis.
The authors summarize the data for anticoagulation for patient with COVID-19 in the inpatient and outpatient settings, patients in the ICU or the floors, with pediatrics and obstetrics, for patients with thrombophilia, those in long-term care facilities, those using anticoagulation prior to COVID-19 hospitalization, and those with prior VTE considering COVID-19 vaccination. More importantly, they assess the weakness of the data supporting their recommendations. While this type of review is not novel, the authors have added some clinically relevant questions and highlighted areas of weakness in the literature. - Venous or arterial thrombosis and deaths among COVID-19 cases: a European network cohort study. 5/16/2022. Burn E. Lancet Infect Dis.
This manuscript reviews with data examining arterial and venous thrombosis from 5 European databases in 909,473 COVID-19 outpatient cases and 32,329 patients hospitalized with COVID-19 from Sept 1, 2020 to July 31, 2021. Only one of the databases was linked to the inpatient database. Risks of venous thromboembolism and arterial thromboembolism were increased with age, among males, and in those who were hospitalized. Their occurrence was associated with excess mortality. Unfortunately, the arterial complications only included CVA and MI and not lower extremity acute thrombosis. These data from the early stages of the pandemic may not be relevant to the present stage. Nevertheless, the scale of this database and its conclusions supporting prior parallel conclusions examining arterial and venous thrombosis in hospitalized COVID-19 patients is worth noting.
May 16, 2022:
- American Society of Hematology living guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID-19: January 2022 update on the use of therapeutic-intensity anticoagulation in acutely ill patients. 5/3/2022. Cuker A. Blood Adv.
These evidence-based guidelines of the American Society of Hematology are an update from the prior guidelines from February 2021 examining the use of therapeutic intensity anticoagulation in acutely ill patients. The panel issued a conditional recommendation in favor of therapeutic-intensity over prophylactic-intensity anticoagulation in patients with COVID-19-related acute illness who do not have suspected or confirmed VTE. The panel emphasized the need for an individualized assessment of thrombotic and bleeding risk. The panel also noted that heparin (unfractionated or low-molecular-weight) may be preferred because of a preponderance of evidence with this class of anticoagulants. This manuscript helps clinicians taking care of COVID-19 with the most up to date information. - Comparative effectiveness over time of the mRNA-1273 (Moderna) vaccine and the BNT162b2 (Pfizer-BioNTech) vaccine. 5/2/2022. Islam N. Nat Commun.
Based on early 2021 data during the Delta surge from over 3.5 million (single Medicare Advantage insurance provider) recipients of 2-dose vaccinations “immunization with mRNA-1273, compared to BNT162b2, provides slightly more protection against SARS-CoV-2 infection that reaches statistical significance at 90 days with a number needed to vaccinate of >290. There are no differences in vaccine effectiveness for protection against hospitalization, ICU admission, or death/hospice transfer (aOR 1.23, 95% CI (0.67, 2.25)).” Approximately 60% received Pfizer and the remainder Moderna vaccines. - Delayed intubation is associated with mortality in patients with severe COVID-19: A single-centre observational study in Switzerland. 4/29/2022. Le Terrier C. Anaesth Crit Care Pain Med.
This is an observational study of 223 adult Covid-19 respiratory failure patients who required intubation between March 2020 and January 2021 and describes the characteristics of ICU patients between two sequential waves of COVID-19 who were cared for with a different management strategy during the second wave. The time from hospital admission to intubation was significantly longer using the second management strategy (4 days vs. 2 days, p < 0.01). All-cause ICU mortality was significantly higher during the second wave (42% vs. 23%; p < 0.01). In a multivariate analysis, the delay between hospital admission and intubation was significantly associated with ICU mortality (OR 3.25, p < 0.05). - Early prolonged prone position in noninvasively ventilated patients with SARS-CoV-2-related moderate-to-severe hypoxemic respiratory failure: clinical outcomes and mechanisms for treatment response in the PRO-NIV study. 4/30/22. Musso G. Crit Care.
In a very detailed study, these Italian authors evaluated the utility of awake prone positioning (PP) in 81 proned and 162 non-proned COVID-19 patients with moderate to severe respiratory failure requiring non-invasive ventilation (NIV). Early prolonged PP (average 12 hours/day) was feasible and was associated with clinical benefits: NIV failure in 17% of PP patients versus 43% of controls, intubation in 11% of PP patients vs 30% of controls, and death in 12% of PP patients versus 36% of controls. Ventilatory, ultrasonographic and biochemical parameters were integrated to individually assess clinical improvements with PP therapy and to provide early signs of NIV failure.
SAB Comment: Though not randomized, this single-center study carefully matched treated and control patients and performed a myriad of statistical and lab/imaging studies which support their findings. Of note is the very long duration of proning in treated patients, exceeding that in other studies of awake PP. - Effectiveness of a COVID-19 Additional Primary or Booster Vaccine Dose in Preventing SARS-CoV-2 Infection Among Nursing Home Residents During Widespread Circulation of the Omicron Variant – United States, February 14-March 27, 2022. 5/5/2022. Prasad N. MMWR Morb Mortal Wkly Rep.
“Analysis … of data from approximately 15,000 skilled nursing facilities found that, compared with primary series vaccination only, an additional or booster dose provided greater protection (relative vaccine efficacy of 46.9%) against SARS-CoV-2 infection during Omicron variant predominance. ….All immune-compromised nursing home residents should receive an additional primary dose, and all nursing home residents should receive a booster dose, when eligible, to protect against COVID-19.” The analysis included 85,494 weekly reports of over 1.1 million residents from 14,758 Skilled Nursing Facilities. Over 90% had received mRNA COVID-19 vaccines. Approximately 22% had received only primary series vaccination, and 65% had received an additional or booster dose. Protection was slightly lower than during the Delta wave. - Hypercoagulability, endotheliopathy, and inflammation approximating 1 year after recovery: Assessing the long-term outcomes in COVID-19 patients. 4/28/22. Fan BE. Am J Hematol.
Sustained hypercoagulability and endotheliopathy have been shown to be present in convalescent COVID-19 patients for up to 4 months after recovery. This study shows that in a significant number of the 39 patients studied with documented prior infection, hypercoagulability, endothelial dysfunction, and inflammation are still detectable approximately 1 year after recovery. D-dimer, Factor VIII, Thrombin generation (Thromboscreen), vWF:Ag, ICAM-1, IL-6 and C-reactive protein were all noted to be elevated in portions (8-49%) of the previously infected patients compared with control patients. Subgroup analysis stratifying patients by COVID-19 severity and COVID-19 vaccination preceding SARS-CoV-2 infection did not show statistically significant differences.
SAB Comment: These observations are noteworthy. However, further study is required to evaluate indications for screening in larger populations, their relationship to delayed complications and ongoing hemostatic management, and whether or how we should modify our perioperative care for post-COVID patients presenting for elective or urgent surgery in the future. - Neurologic Manifestations of Severe Acute Respiratory Syndrome Coronavirus 2 Infection in Hospitalized Patients During the First Year of the COVID-19 Pandemic. 4/25/2022. Cervantes-Arslanian AM. Critical Care Explorations.
In this detailed, prospective, observational study of hospitalized patients (N=16,225) in the registry (VIRUS & COVID-19) from 24 countries, the authors revealed that 12.9% developed serious neurologic manifestations including 10.2% with encephalopathy while stroke, seizure, meningitis/encephalitis were far less frequent at admission. Hospital, ICU & 28-day mortality for patients with neurologic manifestations had OR of 1.51, 1.37,1.58 respectively and had fewer “free days” for ICU, hospital and on ventilator too. Encephalopathy was the driving force for this outcome amongst elderly >72, Blacks with comorbidities. Patients with neurologic manifestations had OR of 4.75 for developing dementia.
SAB Comment: This manuscript had the same problems as other investigators with regards to lack of definition of “Encephalopathy” at admission. - Remdesivir and three other drugs for hospitalised patients with COVID-19: final results of the WHO Solidarity randomised trial and updated meta-analyses. 5/5/22. WHO Solidarity Trial Consortium. Lancet.
Among several “repurposed antivirals” studied in this large 2-year trial, remdesivir emerged as the most promising and Solidarity is the only trial large enough to assess its effects on mortality. This final report subdivides cohorts by degrees of respiratory distress (not on oxygen, receiving oxygen, ventilated) at the time of randomization. Confirming the results of the placebo-controlled ACTT-1 trial which assessed length of stay, remdesivir had no effect on patients already ventilated but had a small effect on mortality in patients on oxygen breathing spontaneously (14·6% versus 16·3%; RR 0·87 [0·76–0·99], p=0·03). These findings are consistent with other reports listed by the COVID-Network Meta-Analysis initiative, a collaborative effort by the WHO and Cochrane.
SAB Comment: This report brings closure to a 2-year effort to assess the benefit of remdesivir in COVID-19 disease and highlights COVID-NMA as a resource for further study and research. - Stress-Related Disorders of Family Members of Patients Admitted to the Intensive Care Unit With COVID-19. 4/25/22. Amass T. JAMA Internal Med.
This is a prospective US multicenter observational study of symptoms of stress-related disorder (PTSD) in 316 family members of patients admitted to the ICU for COVID-19 between February and July 2020. Questionnaires included the short-form Impact of Events Scale 6 (IES-6) for posttraumatic stress disorder (PTSD) and the Hospital Anxiety and Depression Scale (HADS). Screening for PTSD, anxiety and depression was positive in 64%, 45% and 31% of respondents at 3 months and 48%, 34% and 25% at 6 months. Mean IES-6 scores were higher in participants who were female or Hispanic and lower in those with graduate school experience. Respondents with higher IES-6 scores exhibited more distrust of practitioners. The authors cited pre-pandemic studies exhibiting an average incidence of PTSD of 30% in family members. They postulated that COVID-19 ICU visitation restrictions may inadvertently generate a secondary public health crisis through an epidemic of stress-related disorders in families.
SAB Comment: These data were obtained during the first COVID-19 surge in early 2020 and may not represent the incidence of stress-related family disorders in subsequent months or currently. Nonetheless, this study suggests that with the current emphasis on practitioner burnout, attention does need to be given to the impact on family members as well. - Thrombotic and bleeding events, mortality, and anticoagulant use among 546,656 hospitalized patients with COVID-19 in the United States: a retrospective cohort study. 4/30/2022. Deitelzweig S. J Thromb Thrombolysis.
This paper compares 546,656 patients hospitalized with COVID-19 from the Premier Healthcare Database (April 1, 2020 to March 31, 2021) and matched historical controls without COVID-19 (inpatients discharged between April 1, 2018 and March 31, 2019). Overall, the rates of any thrombotic complication and bleeding among patients with COVID-19 were not higher than control patients. Specifically, the VTE event rate and mortality were higher among non-ICU and ICU patients with COVID-19 compared with corresponding controls. Patients with both COVID-19 and thrombotic events had higher mortality than those with COVID-19 without thrombotic events. While these observations are not novel, they do confirm findings from prior smaller datasets and help guide future research.
May 2, 2022:
- Assessment of T-cell Reactivity to the SARS-CoV-2 Omicron Variant by Immunized Individuals. 4/22/2022. De Marco L. JAMA Netw Open.
Omicron BA.1 variant carries >35 Spike protein mutations impacting antibody-mediated neutralization. This study in vaccinated, some also previously infected healthcare workers examined CD4+ and CD8+ responses to key peptides representing the ancestral strain vs the BA.1 variant. CD4+ T-cell numbers reactive with Omicron mutant region peptides showed a 64% decrease vs. similarly located ancestral peptides; CD8+ T cells were 49% reduced. However, T-cell numbers responding to full-length spike peptide libraries were preserved in 100% of subjects, without significant differences between +/- infected groups. Conclusion: robust T-cell responses are maintained to full-length mutated BA.1 Spike and should protect from severe disease.
SAB Comment: BA variants can escape antibody detection. This small study (n=61) in relatively youthful (mean age 41.6) vaccinated heath care workers confirms the successful defensive role CD4+ and CD8+ T-cells should play to maintain clinical defenses against the BA.1 Spike protein. T-cells are critically important and guard against severe disease (hospitalization and death), though not necessarily against an infection. Though these BA.1 data are somewhat reassuring, we await further confirmatory T-cell studies in older populations and how well T-cells from different vaccination status (e.g. boosted vs. non- boosted, etc.) groups fare against the now prevalent BA.2 variant and the newly emerging BA.4 and BA.5 variants. - Clinical characteristics with inflammation profiling of long COVID and association with 1-year recovery following hospitalisation in the UK: a prospective observational study. 4/23/22. The PHOSP-COVID Collaborative Group. Lancet Resp Med.
In this British prospective, longitudinal, cohort study, the proportion of adult COVID-19 patients reporting ongoing symptoms was virtually unchanged between 5 months (74.5% of n=1,965) and 1 year (70.1% of n=807) after hospital discharge. Data was gathered using questionnaires and physiologic testing. Female sex, obesity, and mechanical ventilation for COVID-19 were risk factors for poor recovery. Several inflammatory mediators including IL-6 were increased in individuals with the most severe physical, mental health, and cognitive impairments compared with others. Because data was collected on individuals discharged up to April 18, 2021, the numbers of one-year follow-ups are significantly lower than those 5 months post discharge. - Clinical characteristics, physiological features, and outcomes associated with hypercapnia in patients with acute hypoxemic respiratory failure due to COVID-19—insights from the PRoVENT-COVID study. 3/27/2022. Tsonas AM. J Crit Care.
Summary posthoc analysis of patients invasively ventilated for COVID–19 acute hypoxemic respiratory failure: (1) hypercapnia common; (2) hypercapnic patients had a higher BMI and frequent COPD history; (3) hypercapnic patients, ARDS more often classified severe and VTE was diagnosed more often; (4) hypercapnic patients ventilated with slightly lower VT, higher RR, higher PEEP and ΔP, and more MP over the first days of invasive ventilation; (5) MV was not different, but VR was higher in hypercapnic patients; (6) hypercapnia had an association with a longer duration of ventilation and a longer ICU and Hospital LOS, but not with higher mortality rates. Main differences between hypercapnic and normocapnic patients are severity of ARDS, occurrence of venous thromboembolic events, and a higher ventilation ratio. - Comparing COVID-19-related hospitalization rates among individuals with infection-induced and vaccine-induced immunity in Israel. 4/23/2022. Waxman JG. Nat Commun.
In this retrospective cohort study of over two million Israeli individuals, authors estimate that infection-induced immunity provides superior protection against COVID-19-related hospitalization compared to non-recent vaccine immunity (two vaccine doses at least five months previously) with an incidence rate reduction of 75% and 66%, respectively. Boosted vaccine immunity was superior, however, with an estimated incidence rate of COVID-19-related hospitalization reduced by 89%. Risk reduction in those who had a single vaccine dose plus infection was similar to infection alone (75%). - Effectiveness of mRNA-based vaccines during the emergence of SARS-CoV-2 Omicron variant. 4/27/2022. Sharma A. Clin Infect Dis.
This study, carried out in the VA system database from 12/1/21-3/12/2022, sought to determine the efficacy of the Pfizer and Moderna vaccines against the SARS-CoV-2 omicron variant. Subjects (271,267) having a 3rd dose of Pfizer or Moderna (187,507) were matched to an individual with 2 doses, and an unvaccinated person. The outcomes were infection, hospitalization, and death. As reported in previous studies, outcome was better with three doses of either vaccine than with the primary series alone, than without vaccination. The discussion includes analysis of possible confounding factors and applicability to the general population. - Influence of sex on development of thrombosis in patients with COVID-19: From the CLOT-COVID study. 4/7/2022. Yamashita Y. Thromb Res.
This retrospective, multicenter cohort study enrolled 2894 consecutive hospitalized patients with COVID-19 among 16 centers in Japan from April 2021 to September 2021. They compared thrombotic complications (DVT, PE, arterial thrombosis, MI or ischemic CVA) in men versus women. Even after adjusting for confounding factors in the multivariable logistic regression model, the excess risk for thrombosis of men relative to women remained significant (adjusted OR, 2.51; 95%CI, 1.16–5.43, P = 0.02). These data do confirm prior papers suggesting that men are more prone to thrombotic complications with COVID-19 but do not offer information to change management.
April 25, 2022:
- Association of Early Aspirin Use With In-Hospital Mortality in Patients With Moderate COVID-19. 3/24/2022. Chow JH. JAMA Netw Open.
This observational study (from January 2020 to September 2021) from 64 health systems in the NIH National COVID Cohort Collaborative addressed use of aspirin for hospitalized patients with moderate disease. Of 112,269 patients meeting the criteria for inclusion in the study, 15,272 (13.6%) received a median dose of 81 mg within day 1 of hospitalization. The primary outcome was 28 day mortality, with secondary outcomes being incidence of pulmonary embolism and deep vein thrombosis. Patients who received aspirin had a 28 day mortality OR of 0.85 (p .001, 10.2 vs 11.8) compared to those not given aspirin. They also had fewer PE, but no difference in the rate of DVT. The incidence of GI bleed, cerebral hemorrhage and blood transfusion was not statistically increased. Patients older than 60 and with comorbidity appeared to benefit the most. - Auxora vs. placebo for the treatment of patients with severe COVID-19 pneumonia: a randomized-controlled clinical trial. 4/9/2022. Bruen C. Crit Care.
The CARDEA trial is an industry funded phase 2, randomized, double blind, placebo controlled trial of Auxora, a calcium release-activated calcium (CRAC) channel inhibitor whose active ingredient is CM4620, initially designed to treat acute pancreatitis. The drug blocks proinflammatory cytokine release, including interleukin 6, while preserving endothelial integrity. To determine its effectiveness as an addition to corticosteroids and standard care in treating patients with severe COVID-19 pneumonia, the study enrolled 130 and 131 patients requiring oxygen therapy and having imputed PaO2/FiO2 ratio ≤ 200 to receive Auxora or placebo respectively. Starting in May 2020, patients were followed for 60 days to determine recovery and all-cause mortality. Compared to the placebo group, recovery on Auxora was 3 days shorter (7 vs. 10 days – P = 0.0979) and all-cause mortality on Day 30 and 60 significantly lower (7.7% vs. 17.6% and 13.8 vs. 20.6% respectively. Serious adverse events occurred with lower frequency in the treatment group. The trial, originally designed to enroll 400 patients, was stopped early in the spring 2021 due to declining rates of COVID-19 hospitalizations and increasing use of tocilizumab, a drug prohibited in combination with Auxora by regulatory guidance. The authors consider Auxora safe and worthy of continued clinical development. - Cardiac Complications After SARS-CoV-2 Infection and mRNA COVID-19 Vaccination – PCORnet, United States, January 2021-January 2022. 4/7/2022. Block JP. MMWR Morb Mortal Wkly Rep.
In this CDC report, data from electronic health records in 40 health care systems found that cardiac complications including myocarditis, pericarditis and multisystem inflammatory syndrome were rare but the risk was significantly higher after SARS-CoV-2 infection than after a first or second mRNA COVID-19 vaccination for both males and females in all age groups. Relative risk ratios varied among age, sex, specific complications, and first or second shot, and ranged from 1.8 to 115. The study population consisted of 15,215,178 persons aged ≥5 years, including 814,524 in the infection cohort. Confidence intervals were wide for some estimates due to the rarity of outcomes. - Effect of Antiplatelet Therapy on Survival and Organ Support-Free Days in Critically Ill Patients With COVID-19: A Randomized Clinical Trial. 3/22/22. REMAP-CAP Writing Committee for the REMAP-CAP Investigators. JAMA.
This international RCT randomized 1,557 critically ill COVID-19 patients to aspirin or a P2Y12 inhibitor or no antiplatelet therapy and found no difference in organ support-free days up to 21 days. There was no improvement in survival with antiplatelet therapy. Those randomized to antiplatelet therapy had significantly increased bleeding. This study provides important data for frontline clinicians, and suggests that antiplatelet agents should not be used in critically ill COVID-19 patients.
SAB Comment: The accompanying editorial summarizes the prior RCTs for antiplatelet demonstrating no benefit in noncritically ill patients with COVID-19. - Effectiveness of COVID-19 mRNA Vaccination in Preventing COVID-19-Associated Hospitalization Among Adults with Previous SARS-CoV-2 Infection – United States, June 2021-February 2022. 4/14/2022. Plumb ID. MMWR Morb Mortal Wkly Rep.
A test-negative design was used in this CDC report to estimate effectiveness of COVID-19 mRNA vaccines in preventing subsequent COVID-19–associated hospitalization among adults aged ≥18 years with a previous positive nucleic acid amplification test or diagnosis of COVID-19. Using data from Cosmos, an aggregated data set from electronic health records, and vaccination status, 3,761 hospitalized case-patients were compared with 7,522 matched control-patients. After previous SARS-CoV-2 infection, estimated vaccine effectiveness against COVID-19–associated hospitalization was 47.5% after 2 vaccine doses and 57.8% after a booster dose during the Delta-predominant period, and 34.6% after 2 doses and 67.6% after a booster dose during the Omicron-predominant period. An increasing proportion of the U.S. population has had SARS-CoV-2 infection. COVID-19 vaccination offers additional protection against reinfection leading to hospitalization, with a booster dose(s) offering the highest level of protection. - Fourth Dose of BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Setting. 4/13/22. Magen O. N Engl J Med.
The authors analyzed Israeli data from 182,122 recipients of a fourth vaccine dose, aged over 60 compared with individually matched controls who had received only a third dose at least 4 months earlier. Fourteen to 30 days after the fourth dose effectiveness was 52% against a positive PCR, 61% against symptomatic COVID-19, 72% against COVID-19-related hospitalization, 64% against severe COVID-19, and 76% against COVID-19-related death. Outcomes between groups began to diverge 7 days after the fourth vaccine dose. Because of the recent implementation of fourth vaccine administration, reported outcomes thus far are short-term.
SAB Comment: This study differs from a previously highlighted study from Israel regarding short-term outcomes after a fourth vaccine dose by individually pairing recipients with matched controls and by reporting more outcomes. - Multisystem Involvement in Post-Acute Sequelae of Coronavirus Disease 19. 12/24/21. Novak P. Ann Neurol.
This retrospective study evaluated 9 patients who presented consecutively with chronic fatigue, brain fog, and orthostatic intolerance 10 months following mildly symptomatic COVID-19 infection. Controls included patients with postural orthostatic, tachycardia syndrome (POTS) and healthy participants. Analyzed data included surveys, autonomic assessments (Valsalva maneuver, deep breathing, sudomotor, and tilt tests), cerebrovascular (cerebral blood flow velocity monitoring in middle cerebral artery), respiratory (capnography monitoring), and neuropathic testing (skin biopsies for assessment of small fiber neuropathy) as well as inflammatory/autoimmune markers. Findings for the patients with “post-acute sequelae of coronavirus disease 19” (PASC or Long COVID) included (1) cerebrovascular dysregulation with persistent cerebral arteriolar vasoconstriction; (2) small fiber neuropathy and related dysautonomia; (3) respiratory dysregulation; and (4) chronic inflammation. - Prospective Evaluation of Autonomic Dysfunction in Post-Acute Sequela of COVID-19. 4/5/2022. Jamal SM. J Am Coll Cardiol.
This study of 24 patients with PASC characterized by autonomic dysfunction, described the range of responses to tilt table testing. Testing included provocation using nitroglycerin. Patients with symptoms only after provocation recovered earlier.
April 11, 2022:
- Association of COVID-19 Vaccination in Pregnancy With Adverse Peripartum Outcomes. 3/24/22. Fell DB. JAMA.
Association of SARS-CoV-2 Vaccination During Pregnancy With Pregnancy Outcomes. 3/24/22. Magnus MC. JAMA.
These two retrospective cohort studies address the safety of mRNA vaccines for COVID-19, administered during the last 2 trimesters of pregnancy. Magnus compares 157,521 pregnancies in Norway and Sweden, 18% (28,506) of which underwent vaccination between January 2021 and January 2022. There was no difference in the incidence of preterm birth, stillbirth, small for gestational age babies, decreased Apgar scores and NICU admissions. Fell compares 30,115 unvaccinated and 44,815 pregnancies vaccinated after birth, in Canada (from December 2020 to September 2021) to 22,660 (23%) pregnancies where vaccination occurred mostly in the third trimester. There was no difference in postpartum hemorrhage, chorioamnionitis, cesarean delivery, decreased Apgar scores and NICU admission. Both studies made adjustments for multiple covariants, which produced some differences between the characteristics of the vaccinated and unvaccinated groups. The authors conclude it is safe to administer mRNA vaccines in the second and third trimesters of pregnancy. - Early Outpatient Treatment for Covid-19 with Convalescent Plasma. 3/30/2022. Sullivan DJ. N Engl J Med.
This double-blind, randomized, controlled multicenter US trial compared qualified Covid-19 convalescent plasma in 592 with control plasma in 589, administered within 9 days of COVID-19 symptom onset. The vast majority was unvaccinated. The risk of hospitalization for COVID-19 was less than half as much in the convalescent plasma recipients (2.9% vs 6.3%). - Factors for success of awake prone positioning in patients with COVID-19-induced acute hypoxemic respiratory failure: analysis of a randomized controlled trial. 3/29/22. Ibarra-Estrada M. Crit Care.
Although awake prone positioning (APP) may be helpful, identifying which COVID-19 patients benefit is key. This multicenter, randomized controlled trial from Mexico of 941 COVID-19 patients with respiratory failure requiring high flow nasal cannula (HFNC) between May 2020 and January 2021 identified factors associated with success of APP in preventing intubation. Predictors of APP success in these patients that already required HFNC to maintain SpO2 over 91% included an APP duration at least 8 hours per day, a respiratory rate at enrollment below 26, and improvement in objective measurements (ROX and lung ultrasound) in response to APP. The number needed to treat to prevent intubation was 8.
SAB Comment: This is a post hoc analysis of a meta-trial featured previously in this newsletter. Though some studies do not show a benefit from APP for patients requiring simple nasal oxygen and using APP for shorter periods of time, this study clearly shows that patients with more severe respiratory failure may benefit, especially with longer APP times. Maintaining the prone position in sick, awake patients is difficult. Authors discuss their approach to this challenge. - High-Flow Nasal Cannula Oxygen versus Non-Invasive Ventilation in Subjects with COVID-19: A Systematic Review and Meta-analysis of Comparative Studies. 3/23/2022. Beran A. Respir Care.
This meta-analysis looked at 19 studies (3 randomized clinical trials and 16 observational cohort studies) comparing non-invasive ventilation (NIV; CPAP and BiPAP) with high flow nasal cannula (HFNC) in 3606 COVID-19 patients with respiratory failure. There was no significant difference in the intubation rate and length of hospital stay between HFNC and NIV, despite greater improvement of PaO2/FiO2 ratio with NIV. Although mortality was lower overall in HFNC than in NIV, subgroup analysis of RCTs revealed no significant difference in mortality between HFNC and NIV. - Intravenous methylprednisolone pulses in hospitalised patients with severe COVID-19 pneumonia, A double-blind, randomised, placebo-controlled trial. 4/1/22. Salvarani C. Eur Respir J.
In this multicenter, randomized, double-blind, placebo-controlled trial, 304 hospitalized COVID-19 patients received either 3 boluses of 1 g of methylprednisolone intravenously daily for 3 days or placebo in addition to standard dexamethasone. The key outcome was overall survival in days, discharge without oxygen / ETT. The study group included patients who had < 5 days of symptoms, PaO2: FiO2>200 with O2 and C-reactive protein > 5. Outcomes were similar in both groups including % discharged without O2, duration of hospital or ICU stay, death or adverse reactions. Pulsed methylprednisolone in addition to dexamethasone was safe but redundant to treat hyper-inflammation. - Protection by a Fourth Dose of BNT162b2 against Omicron in Israel. 4/5/22. Bar-On YM. N Engl J Med.
In early January 2022, a fourth Pfizer vaccination dose was offered to persons older than 60 years who had received their third shot at least 5 months previously. This study compares outcomes for 623,355 recipients with 628,966 persons also eligible for the 4th shot. Protection from infection during the ongoing Omicron wave peaked at 4 weeks after 4th shot by a factor of 2 compared with controls, and waned afterward. However, protection from severe disease was greater by a factor of 3.5 and remained durable for the 6 weeks of the study.
April 4, 2022:
- SAB Comment: The following four peer-reviewed vaccine studies add to our overall understanding of mRNA vaccines. Specifically, boosters appear to be very safe; children benefit from vaccination, especially reducing critical COVID; and vaccination and boosters are excellent at preventing severe disease (invasive ventilation and death) even in older people with comorbidities and even during the Omicron phase of the pandemic.
- Boosters reduce in-hospital mortality in patients with COVID-19: An observational cohort analysis. 3/22/22. Mielke N. Lancet Reg Health Am.
This data-rich multicenter observational cohort study of 8,232 adult US COVID-19 patients hospitalized between 8/12/21 and 1/20/22 compared demographic, clinical, and outcome variables in those fully vaccinated and boosted (FV&B), 6%, fully vaccinated (FV), 29%, and unvaccinated (UV), 65%. Although a small number, FV&B patients with breakthrough COVID-19 had lower in-hospital mortality (7.1%) than those FV (10.3%) and UV (12.8%), despite being older and higher risk at baseline. Better outcomes for the FV&B were also found in subgroup analyses of patients older than 65 years and those requiring ICU care; although as expected mortality was higher. - Effectiveness of mRNA Vaccination in Preventing COVID-19-Associated Invasive Mechanical Ventilation and Death – United States, March 2021-January 2022. 3/24/22. Tenforde MW. MMWR Morb Mortal Wkly Rep.
To better evaluate vaccine effectiveness (VE) of both mRNA vaccines in preventing COVID-19 invasive mechanical ventilation (IMV) and death, these authors from 21 US centers used a case control design to study 1440 COVID-19 patients between March 2021 and January 2022. Though vaccinated patients were older, had more comorbidities (especially immunocompromising conditions) than unvaccinated patients, receiving 2 or 3 doses of an mRNA vaccine was associated with a 90% reduction in risk for COVID-19 IMV or death. Protection of 3 mRNA vaccine doses during the period of Omicron predominance was 94%. The authors conclude that COVID-19 mRNA vaccines provide strong protection against severe COVID-19 resulting in respiratory failure or death. - BNT162b2 Protection against the Omicron Variant in Children and Adolescents. 3/30/22. Price AM. N Engl J Med.
In this multicenter study conducted in 23 US states, the authors used a case-control, test-negative design to assess 2-dose BNT162b2 (Pfizer) vaccine effectiveness in preventing hospitalization and critical COVID-19 in 5- through 11- and 12- through 18-year-old patients. Case patients with COVID (n=1185) were compared to control patients (n=1627). Three fourths of subjects were unvaccinated. During the Omicron period, vaccine effectiveness for 12- through 18-year-old patients was 40% against hospitalization, 79% against critical COVID-19, and 20% against noncritical COVID-19. During the Omicron period, vaccine effectiveness against hospitalization among children 5 to 11 years of age was 68%. Ninety-five percent confidence intervals were wide. These figures are lower than during the Delta wave, particularly among adolescents. - Safety and Efficacy of a Third Dose of BNT162b2 Covid-19 Vaccine. 3/23/22. Moreira ED Jr. N Engl J Med.
In this phase 3 industry-sponsored multinational trial, 5,081 participants received a third BNT162b2 (Pfizer) dose and 5,044 received placebo between 7/1/21 and 8/10/21. All had received dose 2 at least 6 months before (median 10.7 months) and almost half had coexisting conditions. After 2 months, among those without evidence of previous SARS-CoV-2 infection, COVID-19 with onset at least 7 days after dose 3 was observed in 6 participants in the vaccine group vs. 123 in the placebo group, a relative vaccine efficacy of 95.3%. Efficacy began at 7 days and was maximal at 14 days. None in either group was hospitalized for COVID-19, supporting ongoing protection from serious disease from the first 2 shots. Injection-site pain was the most frequently reported adverse event. No new safety concerns, including cases of myocarditis or pericarditis, were reported. Serious adverse events were reported by fewer participants in the vaccine group than in the placebo group (0.3% vs. 0.5%).
- Boosters reduce in-hospital mortality in patients with COVID-19: An observational cohort analysis. 3/22/22. Mielke N. Lancet Reg Health Am.
- Dysfunctional breathing diagnosed by cardiopulmonary exercise testing in ‘long COVID’ patients with persistent dyspnoea. 3/31/22. Frésard I. BMJ Open Respir Res.
Cardiopulmonary exercise testing (CPET) identifies mechanisms of dyspnea by simultaneously evaluating cardiovascular adaptation, ventilation and gas exchange through exercise. Dysfunctional breathing (DB) with normal PaCO2 and V̇E/V̇CO2 has been described in long COVID, particularly erratic ventilation with wide, irregular variations of tidal volume and breathing frequency over the progression of effort during CPET. DB evaluated by CPET occurred in 15 of the 51 COVID patients complaining of dyspnea. DB was associated with younger age and previous mild/moderate acute COVID and was present >200 days following infection. DB without hyperventilation with erratic breathing and deep sighs may also explain persisting dyspnea in long COVID patients. The pathophysiology is unknown. A prompt diagnosis is needed in order to offer specific respiratory training. - SARS-CoV-2 Placentitis and Intraparenchymal Thrombohematomas Among COVID-19 Infections in Pregnancy. 3/21/22. Huynh A. JAMA Netw Open.
This research letter describes a retrospective review, with clinical correlation, of 47 placentas infected with SARS-CoV-2 between January 2020 and November 2021. Placentas from 2021 were considered to represent predominantly Delta infection, and were compared to those of 2020. All placentas displayed syncytiotrophoblast necrosis, perivillous fibrin, and intervillositis, but, of the 39 cases from 2021, 29 also had intraparenchymal thrombohematomas. Stillbirth occurred in 72% of patients with thrombohematomas, whereas 17 of 18 placentas without thrombohematomas were associated with live births. Thrombohematomas could be observed on ultrasound.
SAB Comment: This study is of value for its hypotheses-generating potential, as well as to note that all placentas except one came from unvaccinated patients. - Trajectories of Neurologic Recovery 12 Months After Hospitalization for COVID-19: A Prospective Longitudinal Study. 3/22/22. Frontera JA. Neurology.
This study reports the results of telephone psychological testing and interviews of 294 patients 12 months after symptom onset of moderately severe to severe COVID-19 infection. Patients were tested to assess functional status and cognition and given structured interviews to determine presence of anxiety, depression, fatigue and sleep impairment. Abnormal scores on cognitive testing persisted in 50% of patients without a pre-COVID history of cognitive abnormalities, irrespective of the presence or absence of a neurological complication during hospitalization. Rates of abnormal cognition were substantially higher than rates of abnormalities in other domains such as activities of daily living, anxiety, depression, fatigue or sleep. Between the 6- to 12-month evaluations, the majority of patients did not have improvements in functional status or activities of daily living; however, there were significant improvements in cognition and anxiety scores.
March 28, 2022:
- A randomized double-blind placebo-controlled clinical trial of nitazoxanide for treatment of mild or moderate COVID-19. 2/28/2022. Rossignol J. eClin Med.
Nitazoxanide, an approved therapy for intestinal parasitic infections, was administered in this industry-sponsored phase-3 study to 184 mild-moderate COVID-19 patients within 72 hrs. of symptom onset (600 mg p.o. BID x 5 days). Outcomes were compared with 195 who received a placebo. Time to sustained clinical resolution was shortened by a median of 1 day. Progression to severe COVID-19 within 28 days occurred in 1/184 (0.5%) treated patients and 7/195 (3.6%) placebo patients, an 85% relative reduction. Antiviral activity is attributed to a host-directed mechanism targeting key viral protein formation at a post-translational level, and stimulated innate immunity. Larger trials are recommended. - Associations of statin use with 30-day adverse outcomes among 4 801 406 US Veterans with and without SARS-CoV-2: an observational cohort study. 3/19/2022. Wander PL. BMJ Open.
This manuscript examines the outcomes of veterans with ≥1 positive nasal swab for SARS-CoV-2 between 1 March 2020 and 10 March 2021 (cases; n=231,154) and a comparator group of controls comprising all veterans who did not have a positive nasal swab for SARS-CoV-2 but who did have ≥1 clinical lab test for SARS CoV-2 performed during the same time period (n=4,570,252). They examined the effects of statin use with respect to hospitalization, ICU admission and death at 30 days. There was no effect on the use of statins on the outcomes of SARS CoV 2. These data confirm prior RCT data demonstrating the lack of effect but with much larger numbers. - COVID-19-Associated Croup in Children. 3/8/22. Brewster RCL. Pediatrics.
This article from Boston Children’s Hospital describes the change in croup incidence as the COVID-19 variant evolved. During the 22 month-long study, a total of 75 children infected with SARS-CoV-2 entered ER or hospital care due to croup. A 4-fold increase in croup admissions occurred after 12/4/2021 as Omicron became the dominant viral form. During the study, 9 patients were hospitalized, with a median length of stay of 1.7 days. Four patients were admitted to the ICU. No patients died or were endotracheally intubated. Decadron was administered to 97% and all children hospitalized received racemic-epinephrine. Comprehensive viral testing was not available, so viral co-infection could not be excluded. Although rare (75 cases in 100,000 population of children seeking hospital care), the increase in croup was statistically significant during the Omicron surge, according to these authors. - Effects of SARS-CoV-2 on prenatal lung growth assessed by fetal MRI. 3/19/2022. Stoecklein S. Lancet Respir Med.
The authors of this letter to the editor analyzed fetal MRI scans in 34 mothers with uncomplicated SARS-CoV-2 infection during pregnancy to elucidate the effects of the infection on fetal lung development. Fetal lung volume was significantly reduced compared with age-adjusted reference values, in the absence of structural abnormalities or organ infarction, and was not explained by differences in somatic growth. Reductions in lung growth were noted primarily with SARS-CoV-2 infections acquired during the third trimester. Neonatal follow-up in 21 of 34 neonates at birth showed adequate birthweight for gestational age and no indication of acute postnatal respiratory distress. - One-year pulmonary impairment after severe COVID-19: a prospective, multicenter follow-up study. 3/22/22. Faverio P. Respir Res.
In this Italian multicenter, prospective, observational study, 287 patients hospitalized for SARS-CoV-2 pneumonia were stratified by maximum ventilatory support (“oxygen only,” “continuous positive airway pressure” and “invasive mechanical ventilation”) and followed up at 12 months after discharge. At that time, reduced diffusion capacity for carbon monoxide and non-fibrotic interstitial lung abnormalities were common, particularly in older patients who had required higher ventilatory support. Twenty percent of patients showed a distance walked lower than expected, without differences between groups. No patients showed oxygen desaturation or required oxygen supplementation during the test, but mild dyspnea was reported by 35% of patients, again with no differences between groups, compared to 29% at 6-month follow-up.
March 22, 2022:
- Effect of Sotrovimab on Hospitalization or Death Among High-risk Patients With Mild to Moderate COVID-19: A Randomized Clinical Trial. 3/14/2022. Gupta A. JAMA.
Randomized 1057 non-hospitalized patients (symptomatic, mild to moderate COVID-19 + risk factor) study (international, multicenter) conducted August 2020 to March 2021 to evaluate efficacy of single Sotovimab (engineered human monoclonal antibody) infusion to test efficacy in preventing disease progression (all-cause hospitalization lasting >24 hours for acute illness management or death). 5 secondary outcomes tested: all-cause emergency department (ED) visit, hospitalization of any duration for acute illness management, or death through day 29 and progression to severe or critical respiratory COVID-19 requiring supplemental oxygen or mechanical ventilation. All cause hospitalization and 4/5 endpoints statistically reduced compared to placebo; single intravenous dose of sotrovimab, compared with placebo, significantly reduced the risk of a composite end point of all-cause hospitalization or death through day 29. (Study conducted prior to anti-viral introduction.)
SAB Comment: Since their introduction, oral antivirals may be prescribed as a first choice in preventing progression of early COVID disease. Nonetheless, “Sotrovimab retains in vitro activity against the Omicron variant and is expected to provide clinical benefit in patients with Omicron infection.” (Anti-SARS-CoV-2 Monoclonal Antibodies | COVID-19 Treatment Guidelines (nih.gov)) The side-effect profile may favor its use in patients unable to tolerate other options. - Efficacy of a Fourth Dose of Covid-19 mRNA Vaccine against Omicron. 3/17/22. Regev-Yochay G. N Engl J Med.
This research letter from Israel examines the immune response and vaccine efficacy to a 4th dose of mRNA vaccine in 274 young, healthy healthcare workers, compared to matched controls. This non-randomized study took place in January 2022 during an Omicron surge. All subjects were tested with RT-PCR weekly. The 4th vaccine dose increased the antibody levels and viral neutralization by a factor of 10, but vaccine efficacy was low and relatively high viral loads were found, suggesting those with positive RT-PCRs were infectious. The authors conclude that a 4th vaccination of healthy people may have only marginal benefits. Older and vulnerable populations were not assessed.
SAB Comment: Though a small study, this is hopefully the first of much more information on the utility of a second booster. Of note is the infectiousness of those who get Omicron, whether or not they received a 4th vaccine dose (masks may still play a role), and that data on an older population with more comorbidities has yet to be presented. - Neutralization of the SARS-CoV-2 Omicron BA.1 and BA.2 Variants. 3/16/22. Yu J. N Engl J Med.
Omicron has three major sublineages: BA.1, BA.2, and BA.3, each with common and unique mutations to evade neutralizing antibodies (NAbs). Recently, BA.2 is surging. These investigators compared NAbs against Wuhan and BA.1/BA.2 strains induced post-Pfizer vaccinations (primaries + booster, n=24) vs. BA.1 natural infection (n=8). Two weeks postbooster vaccinations, NAbs to each virus rose by 10-fold vs post-primary vaccination; BA.1 NAbs =1.4x BA.2. Two weeks post-BA1 infection, BA.1 and BA.2 titers were each 3x higher vs. postbooster values. Conclusion: BA1 infection confers higher cross-reactive BA.2 NAbs than vaccination alone and the BA.2 surge is from increased infectivity, not immunological escape.
SAB Comment: Neutralizing antibody titers were undetectable to both BA.1 and BA.2 six months postprimary series before receiving boosters. This emphasizes a critical need for the six-month booster to protect against variants. Seven of eight infected patients were previously vaccinated. The unvaccinated eighth rapidly became critically ill. - Palliative care consultation and end-of-life outcomes in hospitalized COVID-19 patients. 12/13/21. Cheruku SR. Resuscitation.
This is a multicenter analysis of end-of-life care for 3,227 adult patients who died from COVID-19 between March 2020 and March 2021 in US hospitals without resource constraints, based on registry data of the Society of Critical Care Medicine. Cardiopulmonary resuscitation was given to 10% of patients and not given to 90%; about 20% of both groups had a palliative care consultation. However, patients who received individualized comfort care measures rather than continuation of life-sustaining treatment were significantly more likely to have had palliative care consultation (43.2% v 8.5%). The authors suggest that palliative care consultation at the end-of-life may better align the needs and values of patients with their received care. - The Fragility of Statistically Significant Results in Randomized Clinical Trials for COVID-19. 3/18/22. Itaya T. JAMA Netw Open.
The objective of this study was to use the fragility index (FI) to evaluate the robustness of statistically significant findings from RCTs for COVID-19. Forty-seven English language articles published by August 7, 2021, involving 138,235 participants were included. In order to apply the index, studies must randomly assign patients 1:1 into 2 parallel groups and reported at least 1 binary outcome as significant in the abstract. In this study, many randomized clinical trials (RCTs) had a low FI, challenging confidence in the robustness of the results. The median was 4, meaning a change in outcome of only 4 participants was required to change the analysis findings from “statistically significant” (P<0.05) to not significant. In over half of the trials, the FI was less than 1% of sample size. Thirty-six were drug trials, with a median FI of 2.5. The most robust group contained 6 vaccine trials, with a median FI of 119. Authors state, “The fragility of RCT results should be considered before applying them to clinical settings,” and “Health care professionals and policy makers should not rely heavily on individual results of RCTs on COVID-19,” particularly small studies.
March 14, 2022:
- Assessing clinically meaningful hypercoagulability after COVID-19 Vaccination: a longitudinal study. 3/7/2022. Campello E. Thromb Haemost.
This article reviews the hypercoaguable state induced by COVID-19 vaccination. Volunteers awaiting vaccination with either the AstraZeneca or Pfizer vaccine were enrolled. Venous samples were obtained before vaccination and at 3±2 days (T1) and 10±2 days after the vaccine (T2). Coagulation monitoring was assessed via platelet count, whole blood thromboelastometry and impedance aggregometry, plasma thrombin generation and anti-PF4/heparin IgG antibodies. 122 subjects were enrolled equally between the two vaccines. The AstraZeneca cohort showed a slight but transient increase in thrombin generation at T1, which promptly decreased at T2. In addition, the second dose of either vaccine was associated with increased thrombin peak. PF4/heparin antibodies demonstrated stable titre through T1 and T2. No relevant differences were detected in platelet count and aggregation, or thromboelastometry parameters. No thrombotic or haemorrhagic events occurred. The authors conclude that no clinically meaningful hypercoagulability occurred after either vaccine, albeit keeping in mind that thrombin generation may increase in the first days after the second dose of either vaccine and after the first dose of the AstraZeneca vaccine. - Characteristics and Outcomes of COVID-19 Patients Supported by Venoarterial or Veno-Arterial-Venous Extracorporeal Membrane Oxygenation. 3/7/2022. Haroun MW. J Cardiothorac Vasc Anesth.
This review of VA and VAV ECMO for COVID-19 included 37 patients from 12 centers who were admitted between 3/01/2020 and 4/30/2021, with final follow up at 90 days on 7/31/21. Patients were grouped as survivors or non-survivors, with no control group. Mortality was 65%. A longer duration between initiation of IMV and ECMO initiation, higher BMI and higher C-reactive protein were noted in the non-survivor group. - Clinical severity of, and effectiveness of mRNA vaccines against, covid-19 from omicron, delta, and alpha SARS-CoV-2 variants in the United States: prospective observational study. 3/10/2022. Lauring AS. BMJ.
Using a test-negative case-control design, this study prospectively evaluated mRNA vaccine effectiveness (VE) in 21 U.S. hospitals for 11690 adult patients admitted to the hospital, 5728 with COVID-19. The time period (March 2021 to January 2022) included inpatients with alpha, delta and omicron infections. mRNA vaccines were found to be highly effective in preventing COVID-19 associated hospital admissions for all variants, but three vaccine doses were required to achieve protection against omicron (VE =86%) similar to the protection that two doses provided against the delta and alpha variants (VE = 85-94%). Among adults admitted to hospital with COVID-19, the omicron variant was associated with less severe disease than the delta variant but still resulted in substantial morbidity (15% invasive ventilation) and mortality (7%). Vaccinated patients admitted to hospital with COVID-19 had significantly lower disease severity than unvaccinated patients for all the variants. - Covid-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. 3/6/2022. Andrews N. N Engl J Med.
Using a test-negative case-control design, researchers from England looked at over 2.5 million PCR tests in symptomatic patients to determine vaccine effectiveness (VE) against delta and omicron variants. Various combinations of the available vaccines (BNT162b2-Pfizer, mRNA-1273- Moderna and ChAdOx1 mCoV-19- Astra-Zeneca) were evaluated. All vaccines showed reduced VE for omicron compared with delta, especially months after only two doses. A booster with either BNT162b2 or mRNA-1273 restored VE to the 65-75% range, which also began tapering off 4 weeks later. They were unable to evaluate VE against severe disease. - Mechanically ventilated patients shed high titre live SARS-CoV2 for extended periods from both the upper and lower respiratory tract. 3/1/22. Saud Z. Clin Infect Dis.
Secretions from 25 mechanically ventilated COVID patients at the University Hospital of Wales were tested for viral RNA and infectious virions in early 2021. One hundred seventeen samples (44 saliva, 32 subglottic above ETT cuff, 41 bronchoalveolar lavage [BAL]) showed extremely high rates of positive qPCR across all sample types, however live virus was most common in saliva (68%) and least common in BAL (32%). Average titers of live virus were highest in subglottic aspirates. SARS-CoV-2 shedding typically ceases beyond 10 days from symptom onset; however, 14/25 studied patients shed live virus for >20 days and one for 98 days. “This information is important for decision making around cohorting patients, de-escalation of PPE, and undertaking potential aerosol generating procedures.” - Outpatient Pulmonary Rehabilitation in Patients with Long COVID Improves Exercise Capacity, Functional Status, Dyspnea, Fatigue, and Quality of Life. 2/24/2022. Nopp S. Respiration.
In this study 58 patients (mean age 47 years, 43% women, 38% severe/critical COVID-19) were included in the per-protocol-analysis of the results of a multidisciplinary pulmonary rehab program. At baseline (i.e., in mean 4.4 months after infection onset), mean 6MWD was 584.1 m and functional impairment was graded at a median at 2 on the post Covid functional status (PCFS, scored 0-4). On average, patients improved their 6MWD by 62.9 meters and reported an improvement of 1 grade on the PCFS scale. Symptoms improved including presence of dyspnea (p < 0.001), fatigue (p < 0.001), and quality of life (p < 0.001). Also, pulmonary function parameters (FEV1, lung diffusion capacity, inspiratory muscle pressure) significantly increased during rehabilitation. - Prolonged unconsciousness is common in COVID-19 and associated with hypoxemia. 3/7/22. Waldrop G. Ann Neurol.
In this multi-center, retrospective follow-up of 795 ICU patients from two waves of the COVID pandemic, the median time to recovery of command-following (RCF) was 30 days following the initiation of mechanical ventilation. The study group was limited to those patients who had a Glasgow Coma Score < 6 on the 7th day of endotracheal intubation. Median time to RCF increased by 16 days for patients with at least one episode of PaO2 ≤55mmHg (p<0.001). Time to RCF increases with duration of hypoxemia after adjusting for known confounders including sedation and does so even among patients without brain imaging abnormalities. The authors state “These observations should be taken into account when making decisions about life-sustaining therapies.” - SARS-CoV-2 is associated with changes in brain structure in UK Biobank. 3/7/22. Douaud G. Nature.
This longitudinal study compares brain scans from the UK Biobank in 401 participants who had COVID-19 to 384 matched controls who were negative for SARS-CoV-2. The scans were done 38 months apart, and an average of 141 days post-COVID-19 diagnosis. Hundreds of brain-imaging derived phenotypes (IDPs) were measured and analyzed. Compared to controls, viral exposure was associated with parahippocampal and orbitofrontal grey matter reduction, greater changes in markers of tissue damage in regions functionally connected to the olfactory cortex, greater reduction in global brain size and larger cognitive decline between scans. The finding remained when patients hospitalized for COVID-19 were removed. The average percent of change compared to control (-0.2 to -2%) was considered moderate by the authors. The study is ongoing. - The immunology and immunopathology of COVID-19. 3/10/22. Merad M. Science.
On the two-year anniversary of the COVID-19 pandemic, these authors summarize progress in understanding immune mechanisms that lead to clinical expression of acute symptomatic and asymptomatic disease. They also detail immune mechanisms in the symptom prolongation involved in “Long COVID syndrome” (aka, post-acute sequelae of SARS-CoV-2, PASC). Viral entry is traced from initial innate mechanisms to adaptive mechanisms followed by resultant pathophysiology. Risk factors and current therapies intersecting immune mechanisms are discussed.
SAB Comment: This up-to-date review is very well written, cites key references and provides a state-of-the-art understanding of the pathophysiology of SARS-CoV-2 infection and its consequences. As such it will also be helpful to readers who decide to read the original paper on whole genome sequencing reviewed below. - Whole genome sequencing reveals host factors underlying critical Covid-19. 3/7/22. Kousathanas A. Nature.
This basic research study sought to find disease mechanisms by comparing whole genome sequencing (WGS) in 7,491 critical COVID-19 cases vs. 48,400 controls in 224 international ICUs. Twenty-three independently validated variants predisposed to critical COVID-19 including genes involved in interferon signaling, leucocyte differentiation, and blood type secretor status. Multiple genes supported causal roles including myeloid cell adhesion molecules and coagulation factor F8, druggable targets. Conclusion: Though complex and requiring multiple genetic comparator controls, WGS is a highly efficient method to detect therapeutically relevant mechanisms in critical COVID-19 including failure to control viral replication and increased tendencies towards pulmonary inflammation and intravascular coagulation. - Tixagevimab and Cilgavimab (Evusheld) for Pre-Exposure Prophylaxis of COVID-19. 1/25/2022. . JAMA.
This AstraZeneca antibody pair is the first to receive FDA emergency use authorization (EUA) for COVID-19 prophylaxis without known exposure in individuals >12 yrs of age with a history of severe allergy to vaccination or severe immune compromise. Mean antibody half-lives are 83-88 days. The EUA was based on the results of an unpublished double-blind trial (PROVENT) that demonstrated a hazard ratio of 0.23 for symptomatic COVID-19 in 3,448 adults vs. 1724 controls during the Delta wave. There were no severe/critical COVID-19 events in the Evusheld group vs. 5 in the placebo group. (Two other antibody pairs are authorized for post-exposure prophylaxis. There is no clinical data during the Omicron wave.) The combination has decreased neutralizing activity in vitro against the Omicron variant (by 12- to 30-fold vs the ancestral virus); the clinical significance of this difference remains to be determined, as Omicron was not prevalent during clinical trials of Evusheld.
March 4, 2022:
- Association of SARS-CoV-2 Infection With Serious Maternal Morbidity and Mortality From Obstetric Complications. 2/7/2022. Metz TD. JAMA.
In this 2020 comprehensive retrospective study regarding the Composite List (CL) of major complications (including pregnancy induced HBP, infection, bleeding but excluding pulmonary) fetal and maternal morbidity outcomes (n=2352 COVID-19 +ve vs. n=11752 -ve patients) were reported. An increased percentage of CL complications for COVID-19 patients were reported 13.4% vs 9.2% and the authors also noted that those with severe disease (n=586) experienced worse outcomes (26.1% Vs 9.2%). This was likely due to a higher C-section birth rate (45.4% vs 32.4%), a delayed treatment for HBP of pregnancy, infection, PPH, and coagulation disorders due to the pandemic which contributed to the outcome. An increased risk of preterm delivery and NICU admission was reported as well, possibly disease-related induced pathology. - Effect of prone positioning on survival in adult patients receiving venovenous extracorporeal membrane oxygenation for acute respiratory distress syndrome: a systematic review and meta-analysis. 1/17/2022. Papazian L. Intensive Care Med.
This is a meta-analysis of 13 studies (1836 patients) published between 2018-21 to evaluate the effect on survival of prone positioning during veno-venous extracorporeal membrane oxygenation (VV-ECMO) in patients with acute respiratory distress syndrome (ARDS). Seven of the studies contained COVID-19 patients only, and all but one were observational. 28-day survival was 74% with and 58% without prone positioning (p<0.0001). The benefit of prone positioning did not appear to be different between studies on COVID-19 and non-COVID-19 patients.
SAB Comment: The limitations inherent in this heterogenous selection of largely observational studies highlights the challenge of performing randomized controlled studies in such severely ill patients. Nonetheless this meta-analysis further informs the benefit of prone positioning in patients on VV-ECMO for severe ARDS, whether or not it is due to COVID-19. - ‘I can’t cope with multiple inputs’: a qualitative study of the lived experience of ‘brain fog’ after COVID-19. 2/12/22. Callan C. BMJ Open.
The authors collected and analyzed comments from 50 patients describing their sensations living with neurocognitive dysfunction resulting from “long COVID.” Qualitative analysis revealed the following themes: rich descriptions of the experience of neurocognitive symptoms (especially executive function, attention, memory and language), accounts of how the illness fluctuated — and progressed over time; the profound psychosocial impact of the condition on relationships, personal and professional identity; self-perceptions of guilt, shame and stigma; strategies used for self-management; challenges accessing and navigating the healthcare system; and participants’ search for physical mechanisms to explain their symptoms.
SAB Comment: The analysis is supplemented with a series of direct quotes from some of the study subjects which may give valuable insight to clinicians caring for similar patients. - Newly diagnosed diabetes vs. pre-existing diabetes upon admission for COVID-19: Associated factors, short-term outcomes, and long-term glycemic phenotypes. 2/4/2022. Cromer SJ. Journal of Diabetes and Its Implications.
This retrospective review of 1902 first-wave COVID inpatients from Massachusetts General Hospital found diabetes in 31% (n=594) of whom 77 had newly diagnosed diabetes mellitus (NDDM). Compared with pre-existing DM, NDDM was associated with younger age, higher inflammatory markers, lower insulin requirements, longer length of stay and intensive care unit admission, but not death. Of 64 NDDM survivors at a median follow-up of 323 days, 56% continued to have DM, and 41% regressed to normoglycemia or pre-diabetes. Stress hyperglycemia is proposed as a major physiologic mechanism. Patients may have received dexamethasone. - Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19. 2/16/22. Hammond J. N Engl J Med.
This industry-sponsored, phase 2-3, double-blind, randomized, controlled trial treated patients with either nirmatrelvir and ritonavir (Paxlovid, n=1039) or placebo (n=1046). Patients were symptomatic less than 5 days, unvaccinated, not hospitalized at enrollment and at high-risk. At 28 days, combined hospitalizations and deaths were 88% lower in the treated group. No deaths were reported in the treated group. Fewer serious adverse events and adverse events leading to discontinuation occurred with nirmatrelvir and ritonavir than with placebo. The most frequent adverse events occurring more often in recipients of nirmatrelvir plus ritonavir were dysgeusia, diarrhea and vomiting.
SAB Comment: This is the definitive study on the use of Paxlovid. See highlights from the NIH therapeutic guidelines for possible drug interactions. - Protection against the Omicron Variant from Previous SARS-CoV-2 Infection. 2/9/2022. Altarawneh HN. N Engl J Med.
This research letter presents data from the relatively young population of Qatar in a case-controlled study of the relative protection of previous SARS-CoV-2 infection against reinfection, comparing 4 variants. The effectiveness of previous infection in preventing reinfection was estimated to be 90% against the alpha variant, 86% against the beta variant, 92% against the delta variant, and 56% against the omicron variant. None of the reinfections progressed to critical or fatal Covid-19. Median intervals between infection and PCR testing among controls and cases ranged from 254 to 314 days for the different variants. - Thromboprophylaxis in Patients with COVID-19. A Brief Update to the CHEST Guideline and Expert Panel Report. 2/15/22. Moores LK. Chest.
This article updates the CHEST guidelines for thromboprophylaxis for COVID-19. Briefly, they suggest full dose anticoagulation with heparin for acutely ill hospitalized patients not in the ICU who are at high risk for deep vein thrombosis and without high risk for bleeding. The remainder of hospitalized patients should be on prophylactic doses of heparin. They do not suggest intermediate doses of heparin. These guidelines are developed using a Delphi approach. In addition, they summarize the evidence supporting these conclusions. This concise report represents the most up-to-date guidelines and is useful for clinicians.
SAB Comment: This manuscript is based upon the evidence (RCTs) that have demonstrated improved mortality and decreased need for organ support with these protocols. The authors do take into account the implementation of these guidelines.
February 28, 2022:
- ASA/APSF joint statement on elective surgery/procedures and anesthesia for patients after COVID-19 infection. 2/2/22. ASA & APSF.
Data from 2020, before vaccine availability, revealed a relative 30-day mortality risk ratio of 2.3 to 3.1 compared to normal following elective surgery performed up to 7 weeks post COVID-19. To minimize postoperative complications, the ASA/APSF recommendation is to delay elective surgery in the unvaccinated for at least 7 weeks following documented COVID-19, and potentially longer if symptoms persist. Data are inconclusive for vaccinated patients and later variants. If surgery is scheduled over 90 days after a COVID-19 diagnosis, a new preoperative PCR test is recommended. Unless new symptoms occur, preoperative PCR testing is not recommended within 90 days of COVID-19, due to the potential for persistent positive tests. - Association of COVID-19 Acute Respiratory Distress Syndrome With Symptoms of Posttraumatic Stress Disorder in Family Members After ICU Discharge. 2/18/22. Azoulay E. JAMA.
This is a prospective 2020 cohort study in 23 French ICUs. Family members of patients with COVID-19 ARDS had a significantly higher prevalence of symptoms of posttraumatic stress disorder (PTSD), anxiety, and depression at 90 days after patients’ discharge from the ICU than family members of patients with non–COVID-19 ARDS. Three hundred and seven patients and 602 family members participated. Twenty-six percent of patients died before the relatives had the day-90 assessment, similar for patients with and without COVID-19. PTSD symptoms were significantly higher in families of patients who died from COVID-19 compared with non-COVID-19 ARDS. Non-COVID-19 ARDS and COVID-19 ARDS survivors had rates of symptoms that were not significantly different for PTSD, anxiety, or depression. Compared with family members, ICU survivors reported fewer PTSD symptoms. The discussion addresses outcome determinants. - Early Administration of Remdesivir and Intensive Care Unit Admission in Hospitalized Pregnant Individuals With Coronavirus Disease 2019 (COVID-19). 2/8/22. Eid J. Obstet Gynecol.
A single-center, retrospective study compared outcomes in 24 pregnant patients who received remdesivir initiated less than 7 days (mean 3 days) from onset of patient-reported symptoms with 17 pregnant patients who received remdesivir initiated 7 or more days from symptom onset (mean 9 days). Patients in the early group were less likely to be admitted to the ICU (21% vs 59%), or to progress to critical disease (12% vs 41%). Additionally, those in the early group had shorter hospital stays (5 days vs 11).
SAB Comment: This result is in line with the recent NIH recommendation that includes remdesivir treatment for COVID-19 patients with elevated risk but not requiring hospitalization or supplementary oxygen. See an overview of the NIH guidelines here. - Extracorporeal membrane oxygenation in coronavirus disease 2019: A nationwide cohort analysis of 4279 runs from Germany. 2/18/22. Friedrichson B. Eur J Anaesthesiol.
A country-wide review of ECMO used in 4,279 COVID-19 patients over an 18-month period ending September 2021 revealed an overall in-hospital mortality of 72% for VA ECMO (291/404) and 65.9% for VV ECMO (2,552/3,875). This contrasted with a previous report of 53% mortality. In those over 60 (43.2%, n=1848), hospital mortality rate was 72.7% for VA ECMO (n=172) and 77.6% for VV ECMO (n=1,301). For those under 60, mortality was 71.6% for VA ECMO (n=166) and 56.9% for VV ECMO (n=1251). Only 7.1% of 14 VV ECMO patients over 80 survived. Intracranial complications, cardiac arrest and renal failure were significant associated findings. Dialysis was associated with a 3-fold mortality. The authors conclude that, “An unconditional recommendation cannot be given for COVID-19,” particularly for those of advanced age. - Frequency of Adverse Events in the Placebo Arms of COVID-19 Vaccine Trials: A Systematic Review and Meta-analysis. 1/18/22. Haas JW. JAMA.
The authors conducted a meta-analysis to investigate the frequency of adverse events (AEs) in placebo groups of 12 COVID-19 vaccine trials that included more than 45,000 participants, equally divided between vaccine and placebo recipients. There were significantly more systemic AEs in vaccine recipients, especially after the 2nd dose. However, about one-third of placebo recipients experienced at least one AE and the placebo arms accounted for 76% and 52% of systemic AEs after the 1st and 2nd doses respectively. These “nocebo” responses included headache, fatigue, malaise and joint pain and were similar to responses in patients receiving vaccine especially after the 1st dose. The authors suggest that informing the public about nocebo responses could decrease anxiety about vaccine side effects and vaccine hesitancy. - Noninvasive respiratory support for COVID-19 patients: when, for whom, and how? 1/15/22. Sullivan ZP. J Intensive Care.
This is a directed review of non-invasive respiratory support (NIRS) for patients with COVID-19 based upon an extensive evaluation of its literature with and without proning (57 citations). Under the NIRS rubric the authors include high flow nasal cannula (HFNC), continuous positive airway pressure (CPAP), and non-invasive ventilation (NIV) which predominantly implies bilevel positive airway pressure (BiPAP). They discuss the rationale and evidence for NIRS in enhancing outcome, particularly avoidance of invasive mechanical ventilation (IMV), in patients with COVID-19 but also compare its use in prior pandemics (SARS, MERS, H1N1) and other forms of acute respiratory failure. They use this to generate a COVID-19 NIRS decision algorithm that includes indications, contraindications, most appropriate form of NIRS, monitoring, steps to reduce health care worker viral exposure and predictors of failure. The authors conclude that judicious use of NIRS may provide an acceptable alternative to early IMV in COVID-19 patients with mild to moderate acute respiratory failure.
SAB Comment: This is an excellent overview that provides a practical summary of the different forms of NIRS, their historic and COVID-19 evidence basis, and a coherent step-wise clinical guideline to decision-making in their application and transition to IMV. As such it provides an extremely useful resource for providers who care for hospitalized COVID-19 patients. - Pulse oximeters’ measurements vary across ethnic groups: An observational study in patients with Covid-19 infection. 1/28/22. Crooks CJ. Eur Respir J.
This research letter from Great Britain addresses the accuracy of pulse oximetry, with regard to skin pigmentation, when oxygen saturation is low. From electronic records, 5374 arterial blood gas oxygen (ABG) saturation results were compared to pulse oximetry saturations obtained within 30 minutes of each other. The study was carried out between February 2020 and September 2021, involved 2997 patients who had suspected or confirmed SARS-CoV-2 and were not in the ICU. Ethnicity, reported as White, Black, Asian, or Mixed, was obtained from the record; the number of non-White patients was small. Pulse oximetry underestimated ABG-determined oxygen saturations greater than 95%, and overestimated low saturations. When ABG saturations were 85-89%, pulse oximetry was higher by 2.4% in Whites, 3.9% in Blacks, and 5.8% in Asians. The authors note greater pulse oximetry inaccuracy in southeast Asians as well as Blacks.
SAB Comment: The statistics here are presented as a reminder of limited accuracy of pulse oximetry, especially isolated measurements. Its value lies in providing trends with continuous monitoring. - Respiratory mucosal delivery of next-generation COVID-19 vaccine provides robust protection against both ancestral and variant strains of SARS-CoV-2. 2/18/22. Afkhami S. Cell.
Using both a human and chimpanzee adenoviral (Ad) vectored vaccine expressing three COVID antigens (spike-1, and internal/conserved nucleocapsid, and RNA-dependent-RNA-polymerase) these investigators showed marked protection in a murine model. Single-dose intranasal was much superior to IM immunization. Tripartite protective immunity was demonstrated in local and systemic antibody responses, mucosal tissue-resident memory T cells and mucosal-trained innate immunity. Intranasal immunization protected against the ancestral strain and two variants of concern (VOC): alpha (UK) and beta (S. African). Conclusion: Intranasal mucosal delivery of this Ad-vectored multivalent vaccine is a next-generation COVID-19 vaccine strategy to induce all-around mucosal immunity against current and future VOC.
SAB Comment: This scientific tour de force used a murine model to rigorously demonstrate the superior protective effects of their trivalent vaccine administered intranasally vs. IM. Human phase 1 trials have begun comparing immune responses to both the human and chimpanzee versions after aerosol delivery to mRNA-vaccinated humans (clinicaltrials.gov: NCT05094609). - Risks of mental health outcomes in people with covid-19: cohort study. 2/16/22. Xie Y. BMJ.
This Veterans Administration study carefully documents the mental health outcomes of 153,848 COVID-19 survivors at one year. These outcomes were compared to two control groups, contemporaneous and pre-COVID. The risks of mental health disorders was substantial and spanned several disorder categories, including anxiety, depression, stress and adjustment disorders, opioid and other substance use disorders, cognitive decline and sleep disorders. The risks were evident even among those with COVID-19 who did not require hospital admission. The authors feel that tackling mental health disorders among survivors of COVID-19 should be a priority.
SAB Comment: The authors analyzed an enormous amount of data, and presented the results in a series of graphs and tables which succinctly and clearly summarize the scope of post-COVID mental health problems. An associated opinion by the senior author cautions against dismissing these long COVID mental health problems as psychosomatic. - SAB Comment: Although electrical impedance tomography (EIT) is not yet in widespread use, it is an established technology that provides easily repeatable non-invasive bedside organ function monitoring without radiation exposure. The following two studies demonstrate EIT’s usefulness in quantitating areas of lung collapse, dead space and perfusion, and thereby facilitate description and prediction of the benefit of interventions such as prone positioning in severe COVID-19 ARDS.
- Lung-Dependent Areas Collapse, Monitored by Electrical Impedance Tomography, May Predict the Oxygenation Response to Prone Ventilation in COVID-19 Acute Respiratory Distress Syndrome. 12/11/22. Cardinale M. Critical Care Medicine.
The authors conducted an experimental study to determine the correlation between lung-dependent area collapse calculated by electrical impedance tomography (EIT) and oxygenation response (>20% increase in PaO2/FiO2) to prone positioning. In studying 24 patients with severe COVID-19 ARDS, the authors found that dependent lung areas collapse of greater than 13.5% had a 94% positive predictive value for improved oxygenation with prone positioning. They also found a response prediction AUC of 0.94. - Effects of Prone Position on Lung Recruitment and Ventilation-Perfusion Matching in Patients With COVID-19 Acute Respiratory Distress Syndrome: A Combined CT Scan/Electrical Impedance Tomography Study. 2/11/22. Fossali T. Critical Care Medicine.
The authors conducted an experimental study to compare the physiologic effects of supine and prone positioning in 21 patients with severe COVID-19 ARDS (C-ARDS) using computed tomography (CT) scanning and electrical impedance tomography (EIT). They observed that prone positioning induced net alveolar recruitment (dorsal recruitment > ventral derecruitment) without change in compliance. This and other observations suggested that prone positioning decreased the potential for atelectrauma. EIT indicated a decrease in ventral dead space and improved ventilation-perfusion matching. The authors conclude that these physiologic responses may be associated with enhanced protective lung ventilation during prone positioning.
- Lung-Dependent Areas Collapse, Monitored by Electrical Impedance Tomography, May Predict the Oxygenation Response to Prone Ventilation in COVID-19 Acute Respiratory Distress Syndrome. 12/11/22. Cardinale M. Critical Care Medicine.
- Timing of elective surgery and risk assessment after SARS-CoV-2 infection: an update: A multidisciplinary consensus statement on behalf of the Association of Anaesthetists, Centre for Perioperative Care, Federation of Surgical Specialty Associations, Royal College of Anaesthetists, Royal College of Surgeons of England. 2/23/22. El-Boghdadly K. Anaesthesia.
This multidisciplinary update of the guidelines for surgery after SARS-CoV-2 infection comes from several United Kingdom medical organizations, focusing on the Omicron variant. The authors emphasize there is no data yet available regarding surgery after Omicron, and the previous need to wait 7 weeks to avoid any increase in risk stands. Avoiding surgery during active infection, vaccination, preoperative prevention measures, exercise, and hospital prevention measures are emphasized. Risk assessment with a tool such as Surgical Outcome Risk Tool v2 (SORT-2) is recommended along with an instructive example of how to calculate individual patient risk. The possibility that use of local or regional anesthesia may lower risk compared with general anesthesia is also discussed. - Waning 2-Dose and 3-Dose Effectiveness of mRNA Vaccines Against COVID-19–Associated Emergency Department and Urgent Care Encounters and Hospitalizations Among Adults During Periods of Delta and Omicron Variant Predominance — VISION Network, 10 States, August 2021–January 2022. 2/11/22. Ferdinands J. MMWR Morb Mortal Wkly Rep.
This CDC study examines mRNA vaccine effectiveness (VE) in 241,204 emergency department/urgent care (ED/UC) encounters and 93,408 hospitalizations across 10 states between August 26, 2021 and January 22, 2022. During both the Delta and Omicron periods, vaccine effectiveness (VE after receipt of a third dose was always higher than VE following a second dose; however, VE waned with increasing time since vaccination. During the Omicron-predominant period, mRNA vaccination was highly effective against the occurrence of both emergency department/urgent care (ED/UC) encounters (VE = 87%) and hospitalizations (VE=91%) within 2 months after a third dose, but effectiveness declined to 66% for prevention of ED/UC encounters by the 4th month, and 78% for hospitalizations.
SAB Comment: This study shows VE after a third dose of mRNA vaccine is excellent against Omicron, though it does wane somewhat with time. The takeaway is that all persons should stay up to date with recommended vaccinations.
February 14, 2022:
- Anxiety, worry, and job satisfaction: effects of COVID-19 care on critical care anesthesiologists. 1/13/22. Siddiqui S. Can J Anaesth.
This correspondence describes the psychological impact of the COVID-19 pandemic on intensivists in late 2020. An online survey was sent to 1,400 mostly American intensivists, 21% of whom responded. Analysis of the results indicate that the COVID-19 pandemic is associated with a high incidence of generalized anxiety disorder (42%) and an increased sense of burnout among critical care anesthesiologists, particularly in females (73%) and younger physicians. This is balanced by enhanced job satisfaction and a sense of being respected and valued for contributions during the pandemic. Seventy-five percent felt that institutional wellness resources were unhelpful. - Association of Convalescent Plasma Treatment With Clinical Status in Patients Hospitalized With COVID-19: A Meta-analysis. 1/25/2022. Troxel AB. JAMA Netw Open.
This meta-analysis examined 8 international RCTs that collectively enrolled 2369 participants and randomized patients with COVID-19 to treatment with convalescent plasma. The data did not demonstrate a benefit (in terms of respiratory support or mortality) which is consistent with prior data. However, the methodology that was used was somewhat unique using real-time individual patient data pooling from all 8 studies. The techniques that are described offer a blueprint for future trials. - Challenges for the Beleaguered Health Care Workforce During COVID-19. 1/27/22. Cutler DM. JAMA Forum.
This is a brief review of the COVID-19 pandemic-induced challenges to the health care workforce that have culminated in burnout and widespread job resignation. The latter has been particularly prevalent in low-wage occupations such as health aides and licensed practical nurses in home health care and nursing homes. Simultaneous increase or steady health care worker demand in the face of falling supply has resulted in wage increases, but still leaves hospitals and health care workers stressed because of inadequate patient coverage and stalled throughput. The author recommends strategies to reduce burnout, including vaccine mandates, continued reimbursement for telehealth, and billing simplification. - Coronavirus Disease 2019 Temperature Trajectories Correlate With Hyperinflammatory and Hypercoagulable Subphenotypes. 1/31/22. Bhavani SV. Crit Care Med.
This is a fascinating study of 5,903 COVID-19 patients classified by a validated group-based trajectory model using oral temperatures measured during 72 hours following hospital admission and dividing the patients into 4 distinct subphenotypes: hyperthermic slow (25%) and fast (25%) resolvers; normothermic (36%) and hypothermic (15%). Clinical characteristics, biomarkers, and outcomes were compared between subphenotypes. Hypothermics had abnormal coagulation markers, suggesting a hypercoagulable subphenotype. Hyperthermic slow resolvers had elevated inflammatory markers and the highest odds of mortality, suggesting a hyperinflammatory subphenotype at high risk for respiratory failure, shock, and mortality. Authors suggest future work should investigate whether temperature trajectory subphenotypes have differential responses to treatment. Excellent figures and discussion complement the manuscript. - Corticosteroids as risk factor for COVID-19-associated pulmonary aspergillosis in intensive care patients. 1/29/2022. Leistner R. Crit Care.
Of the 522 COVID-19 patients treated in this group of German hospitals from March 15 to December 31, 2020, the 47 patients (9%) who had possible or proven Covid associated pulmonary aspergillosis (CAPA) were compared with 168 matched non-CAPA ICU patients. Dexamethasone was adopted as standard therapy in July 2020. The multivariable analysis showed dexamethasone (OR 3.1, CI95 1.1–8.7) and simplified acute physiology score (OR 1.1, CI95 1.03–1.1) to be independent risk factors for CAPA. - COVID-19 Incidence and Death Rates Among Unvaccinated and Fully Vaccinated Adults with and Without Booster Doses During Periods of Delta and Omicron Variant Emergence – 25 U.S. Jurisdictions, April 4-December 25, 2021. 1/27/22. Johnson AG. Morb Mortal Wkly Rep.
In an effort to determine the effectiveness of vaccination and booster shots during the Delta and Omicron surges, the CDC analyzed data from nearly 7 million COVID-19 cases from April through December 2021. Decreases in case incidence rate ratios for unvaccinated vs. fully vaccinated persons with and without booster vaccine doses were observed when the Omicron variant emerged in December 2021. Protection against infection and death during the Delta-predominant period and against infection during Omicron emergence were higher among booster vaccine dose recipients, especially among persons aged 50 and older. COVID-19 vaccination protected against SARS-CoV-2 infection, even as the Omicron variant became prominent.
SAB Comment: This statistically intricate paper is well summarized by the two graphs of cases and deaths, clearly showing the value of vaccination and boosters, including during the Omicron surge. - Long-term cardiovascular outcomes of COVID-19. 2/8/22. Xie Y. Nat Med.
In this Veteran’s Administration database study, COVID-19 survivors who survived 30 days or more after their first positive test, exhibited increased risks and 12-month burdens of cardiovascular diseases, including cerebrovascular disorders, dysrhythmias, inflammatory heart disease, ischemic heart disease, heart failure, thromboembolic disease and other cardiac disorders. Two key findings: (1) the risks and associated burdens were evident among those who were not hospitalized during the acute phase of the disease; (2) complications and associated burdens were correlated with the severity of the acute phase of COVID-19. This finding suggests that care pathways of people who survived the acute episode of COVID-19 should include attention to cardiovascular health and disease. Excellent description of methods accompanied by helpful tables.
SAB Comment: This VA study has important implications for the risk of perioperative major adverse cardiac events (MACE) in COVID-19 survivors. In comparing more than 150k COVID-19 survivors with more than 11 million controls, the risk of post-acute cardiovascular manifestations was significantly higher in patients who had an ICU admission or were hospitalized than for those who were not hospitalized. These factors should be taken into consideration in the preoperative cardiovascular workup of COVID-19 survivors subsequently presenting for moderate or high-risk surgery. - Mental health symptoms in family members of COVID-19 ICU survivors 3 and 12 months after ICU admission: a multicentre prospective cohort study. 2/1/22. Heesakkers H. Intensive Care Med.
To better understand the impact of a COVID-19 intensive care unit (ICU) admission on family members, this prospective cohort study from ICUs in 10 Dutch hospitals followed 197 family members of surviving ICU COVID-19 patients. Questionnaires were completed by family members at enrollment, 3 months and 12 months. Thirty-eight percent experienced at least one mental health symptom (anxiety, depression, or posttraumatic stress disorder) and 23% experienced two or more mental health symptoms, all significantly higher than baseline. Additionally, family members experienced a reduction in quality of life and an impaired work status. Clinicians, including non-ICU clinicians (e.g., general practitioners), should be aware of the high prevalence of mental health problems among family members of COVID-19 ICU patients, especially in family members with mental health symptoms prior to ICU admission. - Neutralization of SARS-CoV-2 Omicron by BNT162b2 mRNA vaccine-elicited human sera. 1/18/2022. Muik A. Science.
In this industry sponsored study, neutralizing capacity of sera of 51 participants who had received 2 or 3 doses of the Pfizer-BNT vaccine was measured using Wuhan, Beta, Delta, and Omicron pseudoviruses. 21 days following 2 doses, sera had >22-fold reduced neutralizing titers against Omicron compared to Wuhan pseudovirus. One month after the 3rd dose, Omicron-neutralizing titers were increased 23-fold compared to two doses (titers similar to Wuhan-neutralizing titers after 2 doses). The findings were confirmed using live SARS-CoV-2 and a subset of participant sera. These lab data suggest that 3 doses of BNT162b2 may provide superior protection against Omicron-mediated COVID-19 than 2 doses. - Prevalence and Risk Factors of Neurologic Manifestations in Hospitalized Children Diagnosed with Acute SARS-CoV-2 or MIS-C. 12/28/21. Fink EL. Pediatric Neurology.
An established consortium found that of the 1,278 SARS-CoV-2 hospitalized children internationally, 40% suffered neurologic manifestations. Findings in this data dense study concluded that headaches were the most common symptom, yet encephalopathy and seizures were significant. Pre-existing neurologic illness increased the risk 3.48-fold. Metabolic illness also increased risk. Delirium and psychosis were rare. Most children were discharged home; death occurred in 1% of the children. - Risk factors and abnormal cerebrospinal fluid associate with cognitive symptoms after mild COVID-19. 1/19/22. Apple AC. Annals of Clinical Trans Neuro.
US investigators studied cognitive postacute sequelae of SARS-CoV-2 (PASC) after mild COVID in patients who reported (n=22) and did not report (n=10) symptoms using structured interviews, neuropsychological testing, and optional cerebrospinal fluid (CSF) evaluations (53%). Those with cognitive symptoms frequently had delayed onset (43%), a higher number of pre-existing cognitive risk factors (2.5 vs. 0; p=0.03) and abnormal CSF findings (77% vs. 0%; p=0.01) versus controls. Cognitive risk factors and immunologic mechanisms may contribute to cognitive PASC pathogenesis. All participants were enrolled in the Long-term Impact of Infection with Novel Coronavirus (LIINC) study (NCT04362150). Further study is recommended.
SAB Comment: Although a very small study, the findings may point toward areas for future investigation. Abnormal oligoclonal banding patterns were identified in 69% (9/13) of CSF samples from participants with cognitive PASC compared to 0% of cognitive controls (p=0.03). Objective testing of cognitive function by HAND criteria correlated poorly with symptoms, raising many questions regarding subjective vs. objective criteria for cognitive function. - Safety and immunogenicity of a high-dose quadrivalent influenza vaccine administered concomitantly with a third dose of the mRNA-1273 SARS-CoV-2 vaccine in adults aged ≥65 years: a phase 2, randomised, open-label study. 2/1/22. Izikson R. Lancet Respir Med.
These are interim results of an ongoing phase-2 industry-supported trial looking for vaccine reactogenicity in adults older than 65 years up to 21 days after receiving a Moderna mRNA-1273 booster alone, a high-dose quadrivalent flu shot alone, or coadministration of both (around 100 patients each). All had completed a 2-dose mRNA vaccination scheduled at least 5 months previously. Reactiogenicity profiles were similar between the coadministration and mRNA-1273 alone groups, with lower reactogenicity rates after the flu shot alone. No safety issues or immune interference were observed. - Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2. 1/11/22. Liu L. Nature.
The US and Hong Kong authors of this paper conclude that “the Omicron variant presents a serious threat to many existing COVID-19 vaccines and therapies, compelling the development of new interventions that anticipate the evolutionary trajectory of SARS-CoV-2”. They present data on the severely reduced neutralizing capacity of 17 of the 19 monoclonal antibodies tested and sera from convalescent patients from first wave cases (n=10), and fully vaccinated and boosted individuals following Pfizer (n=13), Moderna (n=12), Johnson & Johnson (n=9), and AstraZeneca (n=5) vaccines. Data and discussion regarding specific B.1.1.529 (Omicron) mutations are included in this short report. - The emergence of COVID-19 associated mucormycosis: a review of cases from 18 countries. 1/31/2022. Hoenigl M. Lancet Microbe.
This international review of COVID-19-associated mucormycosis analyzed 80 published and unpublished cases. As with non-Covid-related mucor, diabetes, hyperglycemia, systemic corticosteroids, and immunosuppression are the most common predisposing conditions. Most patients presented with rhino-orbital disease. Overall mortality was 49% but pulmonary, cerebral, and disseminated involvement had a particularly poor prognosis and would require ICU care. Survivors frequently had life-changing morbidities (e.g., loss of vision in 46%). Aspergillus co-infection was found in 11%. New molecular (PCR) tests are increasingly available, but culture is required for determination of anti-fungal sensitivities. Amphotericin B +/- azoles +/- surgical intervention for rhino-orbital disease were usual treatments. - The Impact of the COVID-19 Pandemic on Mental Health, Occupational Functioning, and Professional Retention Among Health Care Workers and First Responders. 12/16/21. Hendrickson RC. Journal of General Intern Med.
The authors conducted an observational survey of 510 US health care workers and first responders at a single time-point between September 2020 and February 2021. The goal was to examine the relationships between COVID-19 occupational stressors and symptoms of posttraumatic stress disorder (PTSD), depression (and suicidality), insomnia and anxiety as well as functional impairment and likelihood to leave the current profession. Stressors included factors related to volume (intensity of patient suffering, long hours), demoralization (futile care, lack of personal protective equipment, inadequate support) and risk (to oneself or family). There was a direct relationship between the intensity of stressors (especially demoralization) and psychiatric symptoms (especially PTSD). Both were more intense in nurses than physicians and in emergency medical services than firefighters or police. More than half the health care workers reported that working in the pandemic decreased their likelihood of remaining in their current field. Based upon their data the authors suggest a number of strategies that could mitigate stressors, especially those related to demoralization.
January 31, 2022:
- Cannabidiol inhibits SARS-CoV-2 replication through induction of the host ER stress and innate immune responses. 1/20/22. Nguyen L. Science Advances.
The authors report that cannabidiol (CBD) and its metabolite 7-OH-CBD, but not THC or other congeneric cannabinoids tested, potently block SARS-CoV-2 replication in in-vitro human lung epithelial cells and in mouse nasal turbinates and lung tissue. In matched groups of human patients from the National COVID Cohort Collaborative, CBD treatment for seizures (100 mg/ml oral solution) had a significant negative association with positive SARS-CoV-2 tests. This study highlights CBD as a potential preventative agent for early-stage SARS-CoV-2 infection and merits future clinical trials. The authors caution against use of nonmedical formulations including edibles, inhalants or topicals as a preventative or treatment therapy at the present time. - Cardiac Dysfunction and Arrhythmias 3 Months After Hospitalization for COVID-19. 1/20/22. Ingul CB. J Am Heart Assoc.
In this data-rich Norwegian study of 204 hospitalized COVID-19 survivors and 204 matched controls the authors conclude: “At 3 months after COVID‐19 hospitalization, patients had mildly impaired RV (right ventricular) function, reduced diastolic function, and mainly preserved left ventricular function compared with matched controls. Premature ventricular contractions and nonsustained ventricular tachycardia were common, but the relation to COVID‐19 is unknown. Patients treated in an ICU had similar cardiac function as those not admitted to an ICU. Persistent dyspnea or fatigue were not found to be associated with cardiac function.” - Multiple Early Factors Anticipate Post-Acute COVID-19 Sequelae. 1/19/22. Su Y. Cell.
These numerous authors present an extensively detailed, longitudinal profiling of 209 COVID-19 patients representing a broad spectrum of acute infection severities along with 457 healthy controls. Patients were studied at clinical diagnosis, during acute disease, and 2-3 months after onset of initial symptoms. Four features present at diagnosis (pre-existing type 2 diabetes, assessments of SARS-CoV-2 RNAemia, EBV viremia, and autoantibodies) predicted the likelihood of Post-Acute Sequelae of COVID-19 (PASC), a syndrome defined by the CDC as a range of new, returning, or ongoing health problems that people can experience four or more weeks following initial SARS-CoV-2 infection (called “Long COVID” by some). The authors note that each of the features associated with PASC in the study can be treated.
SAB Comment: This study demonstrates a statistical association between the 4 features noted and the persistence of symptoms 2-3 months later, but the authors don’t claim to show a causal relationship or predict a response to treatment. They don’t attempt to distinguish these associations from those related to intensive care hospitalization or predict the persistence of the symptoms longer than 2-3 months. - One-Year Multidisciplinary Follow-Up of Patients With COVID-19 Requiring Invasive Mechanical Ventilation. 1/2/2022. Zangrillo A. J Cardiothorac Vasc Anesth.
This observational study from a tertiary care facility in Italy provides 12 month follow up data for 61 survivors of COVID-19 requiring invasive mechanical ventilation, who were hospitalized between 2/25/20 and 4/27/20. Median age was 56, 89% were male, none died, and none were lost to follow up. At 12 months, the patients were offered CT exams of the chest and were administered multiple assessments via telephone. They reported good functional recovery (80%), no DOE (90%), severe anxiety or depression (8%), and not working due to disease (11%). Compared to follow up at 2 months, dyspnea, working status and nutrition had improved; cognitive status, functional ambulation, and psychological well being had not changed. Compared to 3 month CT, median residual lung damage had improved from 17.3% to 7.6%, and total lung volume increased 925 cc. Further results are presented. - SARS-CoV-2 breakthrough infections elicit potent, broad and durable neutralizing antibody responses. 1/20/2022. Walls A. Cell.
It is unknown if Covid infection after vaccination (“breakthrough infection”) elicits durable and protective antibody responses. The antibody responses in subjects infected/unvaccinated (primarily delta) or receiving only “2-shot” vaccination were compared with “3-shot” vaccinated, previously infected/then vaccinated or “breakthroughs”. Compared to infected only, or “2-shot” vaccination, the other three groups had serum binding and neutralizing antibodies markedly more potent, durable, and resilient to variant spike mutations. Conclusion: increasing exposures to SARS-CoV-2 antigen(s) (either infection or vaccination) enhance the quantity and quality of antibody responses.
January 24, 2022:
- Ancestral SARS-CoV-2-specific T cells cross-recognize the Omicron variant. 1/18/22. Gao Y. Nat Med.
In some cases, Omicron, despite escaping antibodies, does not lead to severe disease. These investigators examined Omicron spike-specific CD4+ and CD8+ T cell responses induced by prior infection or BNT162b2 (Pfizer) vaccination. Spike-specific CD4+ T cells that cross-recognized Omicron in previously infected or BNT162b2-vaccinated individuals were 84% and 91%, respectively, and for CD8+ T cells were 70% and 92%. The CD4+ and CD8+ T cells were functionally and phenotypically similar in response to the ancestral strain or Omicron. These data indicate that established SARS-CoV-2 spike-specific CD4+ and CD8+ T cell responses, especially after BNT162b2 vaccination, remain largely intact against Omicron. - Antiplatelet Therapy in Patients With COVID-19-More Is Less? 1/18/22. Spaetgens B. JAMA.
This editorial reviews a multinational prospective trial that randomized 562 non-ICU selected patients with COVID-19 to 1) therapeutic heparin or 2) therapeutic heparin with a P2Y12 inhibitor (ticagrelor 63%, clopidogrel 37%). The number of days free of respiratory or cardiovascular organ support, incidence of death in the hospital, and bleeding complications were the same between the two groups.
These data suggest no additional benefits with the use of P2Y12 inhibitors in the face of full therapeutic anticoagulation for non-ICU patients with COVID-19. These data are consistent with prior data failing to demonstrate benefit of the use of aspirin in COVID-19 patients.
SAB Comment: The trial started on February 26, 2021 but was discontinued due to futility 4 months later. - Debulking SARS-CoV-2 in saliva using angiotensin converting enzyme 2 in chewing gum to decrease oral virus transmission and infection. 11/10/21. Daniell H. Molecular Therapy.
This study describes the development of a chewing gum containing virus-trapping proteins expressly designed to minimize oral virus transmission or reinfection. Salivary glands are primary sites of SARS-CoV-2 replication and saliva from symptomatic or asymptomatic COVID-19 patients contains a viral load proportionate to the severity of symptoms. Contagious airborne droplets are the major form of transmission of the virus, which utilizes ACE2 receptors to enter cells. The authors incorporated biomass plant-created cholera toxin B(CTB)-ACE2 powder into flavored chewing gum and tested its ability in vitro to bind to viral spike proteins, debulk virus from saliva and thus potentially reduce oral transmission. Incubation of CTB-ACE2 microparticles with infected saliva reduced SARS-CoV-2 virus count by >95%. Since the mechanism utilizes the ACE2 receptor rather than blocking specific viral antigens, it has the potential to be effective regardless of the variant genome.
SAB Comment: This is a remarkably novel approach to the amelioration of viral transmission that if proven to be beneficial in vivo could have dramatic effects on COVID-19 contagion. It also creates the potential of allowing millions to chew gum and walk at the same time. - Early Remdesivir to Prevent Progression to Severe Covid-19 in Outpatients. 12/22/21. Gottlieb RL. N Engl J Med.
In this randomized, double-blinded, placebo controlled trial of 3 doses of Remdesivir (N=279 vs. placebo N=283), COVID-19 patients, ages 50+, with symptoms and one co-morbidity were studied. Remdesivir had a 87% lower risk of COVID-19–related hospitalization or death and 81% lower risk of medical visits compared to the placebo at day 28. The drug is considered to be safe in the outpatient setting. This group included patients that were unvaccinated and not on oxygen before the emergence of the Delta variant. - Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients. 12/16/2021. Jayk Bernal A. N Engl J Med.
1433 unvaccinated patients with mild/moderate COVID-19 for less than 5 days, and at least one risk factor for severe illness were enrolled in this multicenter phase 3 random double blind placebo controlled study of molnupiravir from 5/6-11/4/21. Patients received placebo or 800 mg twice daily for 5 days, with a primary outcome of hospitalization or death at 29 days. The study was stopped based on a favorable interim analysis, (7.3% molnupiravir vs 14.1% placebo) but, by the time the stop decision was made, enrollment was almost complete. The final results were less favorable (6.8% molnupiravir vs 9.7% placebo). Multiple analyses of subgroups are presented and possible reasons for the difference between the interim and final results are presented. - Myocarditis and Pericarditis After Vaccination for COVID-19. 8/4/21. Diaz GA. JAMA.
This is a report on postvaccination myocarditis or pericarditis derived from data from 40 hospitals in the Providence healthcare system based in 4 states (WA, OR, MT and CA). Out of more than 2 million individuals receiving at least one vaccination (97% Pfizer or Moderna), there were 20 cases of myocarditis and 37 of pericarditis, a rate of 1.0 and 1.8 per 100k vaccinations, respectively. These represented two distinct self-limited syndromes. Myocarditis patients were mostly young (median age 36 years), male, developing symptoms 3-10 days after the second vaccination. Nineteen of 20 were admitted to hospital and all were discharged after a median of 2 days. All patients had symptom resolution or improvement within 5-40 days of onset. Pericarditis patients were also mostly male, but older (median age 59 years) with more comorbidity, developing symptoms 6-41 days after the first or second vaccination. About half (19/37) required hospital admission with a median stay of 1 day. A minority of both groups required cardiovascular therapy in addition to anti-inflammatory medications.
SAB Comment: This US-based report emphasizes that myocarditis and pericarditis are extremely rare, distinct entities occurring in different age groups, that are self-limited with a benign outcome. This risk should be compared with the risk of developing cardiovascular complications in unvaccinated patients with severe COVID-19. - Perioperative Cardiovascular Considerations Prior to Elective Noncardiac Surgery in Patients With a History of COVID-19. 1/12/22. Rohatgi N. JAMA Surg.
Risk evaluation for patients with preoperative cardiac risk factors for noncardiac surgery is standard anesthesiology practice. COVID-19 is associated with cardiovascular complications including but not limited to myocardial injury, dysrhythmias, and thromboembolism. Following symptomatic recovery, optimal timing for elective surgery is discussed with data supporting a seven-week delay. Standard clinical practice guidelines can be applied to COVID-19 patients with clinical appreciation for disease severity in individual cases. A valuable table is included for reference.
SAB Comment: This opinion piece is timely given the current impact of the Omicron surge, high hospital occupancy and healthcare staff shortages on elective noncardiac surgery. However, its value may lie in stimulating discussion rather than providing a strict guideline. We have insufficient data on postacute cardiovascular manifestations and long COVID after Omicron. In a real-world situation, optimal timing of elective noncardiac surgery may depend on the interaction of institutional constraints, appropriate cardiovascular assessment and the relative “electiveness” of surgery, rather than a fixed delay. - Prone Position in Coronavirus Disease 2019 and Noncoronavirus Disease 2019 Acute Respiratory Distress Syndrome: An International Multicenter Observational Comparative Study. 9/24/21. Camporota L. Critical Care Medicine.
This is a retrospective observational multicenter study from seven ICUs in Italy, the UK and France that compared the response to prone positioning between patients with COVID-19 ARDS (n = 220) and non-COVID-19 ARDS (n = 156). Immediately before proning COVID-19 ARDS patients were more hypoxemic but had greater lung compliance than non-COVID-19 ARDS. A positive response to proning, defined by an increase ≥ 20 mmHg in PaO2/FiO2, occurred in nearly 80% of both groups and was greater the lower the initial PaO2/FiO2, and the earlier proning was commenced after intubation. Overall ICU mortality was 45%, with no significant difference between groups, but improved survival was independently associated with younger age, earlier proning and the oxygenation response to the intervention.
SAB Comment: Although this is a retrospective observational study, its strength is that it does not (again) attempt to establish whether proning is better than non-proning. Instead it explores the difference in response to proning between COVID-19 and non-COVID-19 ARDS patients, and finds that they are equivalent and that early intervention is beneficial in both groups. The COVID-19 patients were from the early pandemic (February-May 2020) so the study does not take into account the potential mortality benefit of steroid therapy that became evidence-based later in 2020.
January 10, 2022:
- SAB Comment: The IARS COVID-19 SAB recommends to readers the following group of opinion articles recently published in JAMA written by several members of a pandemic scientific advisory board that counseled President Biden during his transition. We provide these links not as an IARS SAB endorsement of the views expressed, but to encourage readers to review this reflective and forward-looking discussion at the two-year mark of the COVID-19 pandemic and during the current Omicron variant surge.
- The Pandemic Preparedness Program: Reimagining Public Health. 1/6/22. Adashi EY. JAMA.
- A National Strategy for COVID-19: Testing, Surveillance, and Mitigation Strategies. 1/6/22. Michaels D. JAMA.
- A National Strategy for COVID-19 Medical Countermeasures: Vaccines and Therapeutics. 1/6/22. Borio LL. JAMA.
- A National Strategy for the “New Normal” of Life With COVID. 1/6/22. Emanuel EJ. JAMA.
- The First 2 Years of COVID-19: Lessons to Improve Preparedness for the Next Pandemic. 1/6/22. Nuzzo JB. JAMA.
- An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by therapeutic monoclonal antibodies. 1/4/22. VanBlargan L. Res Sq.
This invitro study compares the ability of existing monoclonal antibodies to SARS-CoV-2 (mAb) to neutralize B1.1.529 (Omicron) versus a COVID-19 variant previously studied by the authors and known to be susceptible to mAb. Structural models were used to compare the Omicron spike to the various mAb structures. Using Vero cells, the focus reduction neutralization test demonstrated that the Regeneron, Lilly, and Celltrion mAbs completely lost the ability to neutralize Omicron. The effectiveness of AstraZeneca monoclonal antibodies was reduced 12 fold. S309 (the parent of sotrovimab) was affected least, but was less potent with cells expressing hACE2. - Characteristics and Outcomes of Hospitalized Patients in South Africa During the COVID-19 Omicron Wave Compared With Previous Waves. 12/30/21. Maslo C. JAMA Netw Open.
This report is from the South African Netcare health system representing 49 acute care hospitals. The authors compared outcomes of 17,200 patients evaluated across four COVID-19 waves (ancestral, Beta, Delta and Omicron variants). Compared with the previous waves, the Omicron wave demonstrated a decreased incidence of hospital admission (41.3 v 68-69%) despite only 24.2% of patients known to be vaccinated. Patients were younger (median 36 years v 59 years with Delta), more likely to be female and less likely to have comorbidities. Compared to the Delta wave, the proportion of admitted patients presenting with an acute respiratory condition (31.6 v 91.2%), requiring oxygen therapy (17.6 v 74%), ICU admission (18.5 v 29.9%) and mechanical ventilation (1.6 v 12.4%) was significantly less during the Omicron than the Delta wave. Median length of stay (3 v 9 days) and hospital mortality (2.7 v 29.1%) were also significantly lower. The authors acknowledge the absence of genotyping (but 95% of variants were Omicron at the time of study) and varying public health restrictions during different waves.
SAB Comment: This is one of a number of databases, and one of the very first peer-reviewed publications, suggesting that the Omicron wave is associated with less severe illness, hospitalization and death. At present it is not clear whether this difference results from enhanced acquired or natural population immunity or innately lower Omicron variant pathogenicity. It is also important to recognize that surge patterns in one country are not directly applicable to another. - Decreased severity of disease during the first global omicron variant covid-19 outbreak in a large hospital in tshwane, south africa. 12/31/21. Abdullah F. Int J Infect Dis.
This retrospective cohort study compared 466 SARS-CoV-2 PCR+ inpatients since 11/14/21 to 3976 PCR+ inpatients during pre-Omicron waves. Deaths and ICU admissions were 4.5% vs. 21.3% (p<0.00001), and 1% vs. 4.3% (p<0.00001); length of stay was 4 vs. ~9 days; and mean age was 39 years during Omicron wave vs. 49 years for previous waves. Admissions peaked and declined rapidly with peak bed occupancy at 51% of highest previous peak. Only 1/3 of patients had COVID-19 pneumonia (45% on oxygen vs. 99% previously). Nearly 2/3 of positive PCR results were incidental to admission for other illnesses. - Efficacy and Safety of NVX-CoV2373 in Adults in the United States and Mexico. 12/15/2021. Dunkle LM. N Engl J Med.
The results of this industry-funded, phase 3 trial of NVX-CoV2373 (Novavax) conducted from 12/27/2020-2/18/2021 in 29,582 volunteers in Mexico and the US are presented. Priority access was given to high risk and diverse persons in this randomized, placebo controlled trial, with a second dose after 21 days. The primary outcome was the incidence of a positive PCR 7 days after the second dose over 3 months (14 of 19714 vaccine and 63 of 9868 placebo recipients), representing a 90.4% efficacy. The vaccine was effective against variants of that period. After 3 months, a crossover procedure permitted vaccination for all, and the study continues for durability and safety. - Immunogenicity, safety, and reactogenicity of heterologous COVID-19 primary vaccination incorporating mRNA, viral-vector, and protein-adjuvant vaccines in the UK (Com-COV2): a single-blind, randomised, phase 2, non-inferiority trial. 12/9/2021. Stuart ASV. Lancet.
This data-rich British study of mixed vaccine dosing is most relevant to unvaccinated populations. Subjects, aged 50-75, were randomized to receive an initial dose of either AstraZeneca (AZ) (n=540) or Pfizer (n=532) vaccine. 8-12 weeks later all were randomized 1:1:1 to receive a second dose; same as prime, Moderna, or Novovax. 28 days later, serology revealed differences in antibody titers to the S-protein, and T-cell immunity (assessed in 60%). Results are complex. Highest antibody to S-protein followed Pfizer/Moderna, AZ/Moderna, Pfizer/Pfizer, however those receiving AZ/Novovax had evidence of most robust cellular immunity. Pfizer/AZ did not meet the criteria for non-inferiority to Pfizer/Pfizer however was still superior to AZ/AZ. An editorial further discusses results and implications. - Neutralization of SARS-CoV-2 Omicron variant by sera from BNT162b2 or Coronavac vaccine recipients. 12/16/2021. Lu L. Clin Infect Dis.
A live virus microneutralization assay was used to evaluate the sera of 25 BNT162b2 and 25 Coronavac recipients against two SARS-CoV-2 omicron variants, (HKU691, and HKU344-R346K) as well as ancestral, beta and delta variants. Sera were obtained 56 days after the first dose of the vaccine. None of the Coronavac recipients had neutralizing antibodies to omicron, whereas 20-24% of BNT recipients did. The geometric mean neutralizing titers of BND recipients against omicron were 36 to 40% reduced from those to the ancestral strain. - Post-ICU Syndrome in a Cohort of COVID-19 Survivors in New York City. 12/22/2021. Weidman K. Ann Am Thorac Soc.
Post intensive Care Syndrome (PICS) is a recognized sequelae of critical illness requiring ICU admission. Specific consequences following COVID admissions requring mechanical ventilation and prolonged stays are unknown. Retrospective cohort study of patients attending an ICU recovery clinic compared with non- attending matched group. Overall PICS prevalence 90% with depressive illness (28%), anxiey (21%), post traumatic stress disorder (PTSD/13%), and cognitive impairment (25%) reported among attendees. Similar results seen in non-attendees. No associations found with ICU LOS, benzodiazepine, steroids or paralytic use. Prevalence of PICS suggests importance of careful follow up post discharge. Non-attendees more likely discharged to SNF’s receiving followup care. - Real-World Effectiveness Of Remdesivir In Adults Hospitalized With Covid-19: A Retrospective, Multicenter Comparative Effectiveness Study. 12/15/21. Garibaldi BT. Clin Infect Dis.
This is a large population study (98,000 patients, 160 hospitals across 21 states), examining the still disputed clinical effectiveness of remdesivir. Compared to matched controls, remdesivir was associated with a statistically significant increase in the likelihood of clinical improvement and survival beyond 28 days in patients on no or low-flow oxygen. Of 36,656 matched individuals, 74.0% who received remdesivir and 68.3% of controls achieved clinical improvement before 28 days with a median time to clinical improvement of 7 days (IQR 5,19) in the remdesivir recipients and 9 days (IQR 5,28) for controls. Results are consistent with the outcome of the ACTT-1 trial and underscore the role of remdesivir in patients with mild respiratory symptoms and its failure to improve advanced disease. - Reduced neutralisation of SARS-CoV-2 omicron B.1.1.529 variant by post-immunisation serum. 12/23/2021. Dejnirattisai W. Lancet.
This research letter from the UK presents preliminary data on live virus neutralization by sera from individuals fully vaccinated with either the AstraZeneca (n=22) or Pfizer (n=21) vaccines comparing neutralization of Omicron with Delta and 2 other previous variants. Pfizer provided better protection against Omicron than AZ however protection from either vaccine was far lower than against Delta. This information may inform booster strategies and the development of future monovalent or multivalent vaccines. - Rivaroxaban versus no anticoagulation for post-discharge thromboprophylaxis after hospitalisation for COVID-19 (MICHELLE): an open-label, multicentre, randomised, controlled trial. 12/18/21. Ramacciotti E. Lancet.
This trial from Brazil randomized 320 patients to receive post-hospital discharge prophylactic rivaroxaban or no anticoagulation (from Oct 8, 2020, to June 29, 2021). 165 patients (52%) had been in intensive care. Patients underwent bilateral lower-limb venous ultrasound and CT pulmonary angiogram at day 35. Rivaroxaban was associated with decreased rates of 1) symptomatic and fatal VTE 0.6% vs. 5% and 2) composite of symptomatic VTE, myocardial infarction, stroke, and cardiovascular death (0.6% vs. 5.6%). Furthermore, the magnitude of events was also decreased (more events were asymptomatic in the anticoagulant group than in the control group). This is the first trial demonstrating benefit of post-discharge prophylaxis.
Disclaimer
The material on this website is provided for informational purposes and does not constitute medical advice. New knowledge is added daily and may change over time. Opinions expressed should not be construed as representing IARS policy or recommendations. References and links to third parties do not constitute an endorsement or warranty by IARS.
The material on this website is provided for informational purposes and does not constitute medical advice. New knowledge is added daily and may change over time. Opinions expressed should not be construed as representing IARS policy or recommendations. References and links to third parties do not constitute an endorsement or warranty by IARS.
International Anesthesia Research Society
