Following declarations from the US Department of Health and Human Services and World Health Organization ending the COVID-19 Public Health Emergency, the IARS COVID-19 Scientific Advisory Board (SAB) concluded its review of the scientific literature about SARS-CoV-2 in August. The SAB has reviewed more than 3,100 journal articles and published 1,076 article reviews over the past 42 months. It has been an enormous commitment from the SAB, and the IARS owes our dedicated physician volunteers a huge debt of gratitude for their unwavering participation in this initiative.
The COVID-19 Scientific Advisory Board has compiled and examined published guidelines and reviews on COVID-19 and have provided a collection of those most helpful and relevant for frontline providers below.
To search by keyword, select Ctrl + F on a PC and Command + F on a Mac. Then, enter keyword and Enter.
Governmental Resources
Non-Governmental Resources
Summaries of Special COVID-19 Topics
Disclaimer
The material on this website is provided for informational purposes and does not constitute medical advice. New knowledge is added daily and may change over time. Opinions expressed should not be construed as representing IARS policy or recommendations. References and links to third parties do not constitute an endorsement or warranty by IARS.
Governmental Resources
US Department of Veterans Affairs
Whole Health System Approach to Long COVID. 8/1/22. Veterans Administration.
This guide describes a whole health system approach to long COVID. It is a PACT (patient-aligned care team) guide from the US Department of Veteran Affairs. The whole health system approach is evidence-informed, multidisciplinary, personalized, and veteran-driven. The guide includes a definition of long COVID and post COVID conditions, current incidences of conditions and recommendations for evaluation and treatment. The guide is meant to be updated periodically.
US Food and Drug Administration (FDA)
Coronavirus Disease 2019 (COVID-19) Resource List. US Food & Drug Administration.
In both English and Spanish and continually updated, this website is extensive but provides access to the latest FDA authorization of drugs, vaccines and testing.
Centers for Disease Control and Prevention (CDC)
Guidance for COVID-19.
An extensive, continually updated website with an enormous amount of information. The hundreds of guidance documents are best navigated with the well-placed search function rather than trying to find what you want on your own.
Morbidity and Mortality Weekly Reports
Review the reports recommended by the SAB here.
National Institutes of Health (NIH)
NIH COVID-19 Treatment Guidelines
Excellent, frequently updated, easily navigated website with expert opinions from a diverse panel of academic COVID-19 experts. The “What’s New” and “Table of Contents” provide rapid access to referenced information on all forms of COVID-19 treatment.
Non-Governmental Resources
International Society of Thrombosis and Hemostasis![]() Good practice statements for antithrombotic therapy in the management of COVID-19: Guidance from the SSC of the ISTH. 7/29/22. J Thromb Haemost. Clin Infect Dis.
Good practice statements for antithrombotic therapy in the management of COVID-19: Guidance from the SSC of the ISTH. 7/29/22. J Thromb Haemost. Clin Infect Dis.
This article is a set of best practice statements for deep vein thrombosis prophylaxis and treatment with COVID-19 from the International Society of Thrombosis and Hemostasis. It is not based upon a systematic review but expert opinion. While much of this is similar to prior guidelines, the special considerations section covering pregnancy, pediatrics, chronic kidney disease, obesity and heparin-induced thrombocytopenia without thrombosis are of value.
ISTH guidelines for antithrombotic treatment in COVID-19. 7/29/22. Schulman S. J Thromb Haemost.
This article is a systematic review and guidelines for deep vein thrombosis prophylaxis with COVID-19 from the International Society of Thrombosis and Hemostasis. It was performed by a panel of experts. Among noncritically ill patients hospitalized for COVID-19, the panel gave a strong recommendation (a) for use of prophylactic dose of low molecular weight heparin or unfractionated heparin (LMWH/UFH) (COR 1); (b) for select patients in this group, use of therapeutic dose LMWH/UFH in preference to prophylactic dose (COR 1); but (c) against the addition of an antiplatelet agent (COR 3). Nine additional recommendations are made. The review is comprehensive and current, and the recommendations are graded. Figure 2 is useful as it summarizes the findings.
Nature
Antibody evasion by SARS-CoV-2 Omicron subvariants BA.2.12.1, BA.4, & BA.5. 7/5/22. Wang Q. Nature.
The authors performed systematic antigenic analyses of Omicron subvariants finding BA.2.12.1 only modestly (1.8-fold) more resistant to sera from vaccinated and boosted individuals than BA.2. However, BA.4/5 is substantially (4.2-fold) more resistant and thus more likely to lead to vaccine breakthrough infections. Among therapeutic antibodies authorized for clinical use, only bebtelovimab retains full potency against both BA.2.12.1 and BA.4/5. Serum neutralization assays used sera from persons who received three shots of mRNA vaccines, others who received mRNA vaccines before or after non-Omicron infection, vaccinated patients with BA.1 or BA.2 breakthrough infection as well as pseudoviruses containing point mutations. The Omicron SARS-CoV-2 lineage continues to evolve, successively yielding subvariants that are both more transmissible and more evasive to antibodies.
SAB Comment: Bebtelovimab (Eli Lilly) has been authorized under EUA since 2/22 for mild-to-moderate COVID-19 in patients older than 12 years and weighing at least 40 kg who are at high risk for progression to severe disease. It is given as a single IV injection, within 7 days of symptom onset. Readers may access current NIH Therapeutic Guidelines, including use of bebtelovimab, on the IARS COVID-19 Published Guidelines and Reviews web page.
Infectious Diseases Society of America (IDSA).
This website provides Infectious Diseases Society of America (IDSA) Guidelines on the Treatment and Management of patients with COVID-19. The IDSA frequently updates the site with their carefully considered and well-organized distillation of current knowledge. In particular, their Summary Table titled, “Overview of COVID-19 Treatment Guidelines” is particularly concise and informative. This IDSA guideline link will be placed in the IARS Published Guidelines and Reviews web page for future reference.
Omicron Variant Patient Profile in South Africa. 12/6/21. SAMRC.
Readers interested in following the progress of the Omicron variant in South Africa, where it was first identified on November 24, 2021, are directed to the website of the South African Medical Research Council (SAMRC). This site provides access to a developing patient profile of the impact of the Omicron surge on hospitalization and outcomes here.
American Society of Anesthesiologists (ASA)
ASA’s Statements and Recommendations on COVID-19. American Society of Anesthesiologists.
This easily navigated COVID-19 resource serves as a statement and guideline website for the American Society of Anesthesiologists and is updated as indicated. Includes 20+ “Statements and Recommendations” that focus on surgery, anesthesia and safety; an extensive “Frequently Asked Questions” section; and a variety of other resources including webinars and podcasts.
American Society of Anesthesiologists (ASA) and Anesthesia Patient Safety Foundation (APSF)
ASA and APSF Statement on Perioperative Testing for the COVID-19 Virus. 6/16/22. ASA & APSF.
SAB Comment: Readers are encouraged to review the full publication. A few key points, including the potential use of community transmission metrics to guide the use of universal testing, are summarized below:
SCREENING: All patients should be screened for COVID-19 symptoms and for close contact to someone diagnosed with COVID-19 in the past 10 days.
TESTING: The use and limitations of PCR for SARS-CoV-2 testing are reviewed. If a patient tests positive, elective surgical procedures should be delayed. Antibody testing is not recommended for perioperative screening.
COMMUNITY TRANSMISSION METRICS: Two metrics, based on cases/100K in the last 7 days and % test positivity in the last 7 days, are available by community on the CDC website. When levels are graded substantial to high, universal use of PCR testing is recommended. If community transmission is low to moderate, the patient is asymptomatic, up-to-date in vaccination and having a lower-risk procedure, facilities could consider a more permissive approach to perioperative testing.
A number of specific situations are reviewed, including cases of immunosuppressed or other patients likely to carry transmissible virus for longer than 10 days after infection.
Society of Critical Care Medicine (SCCM)
https://www.sccm.org/Clinical-Resources/Disaster/COVID19
At this site SCCM offers a variety of COVID-19 resources available without membership, including a Rapid Resource Center for educational materials, Surviving Sepsis Campaign COVID-19 Guidelines, COVID-19 journal articles from SCCM publications, and complimentary online training in critical care for non-ICU clinicians.
Elsevier
Elsevier COVID-19 Healthcare Hub
A well-organized collection of clinical resources focused on the latest evidence-based practices, covering symptom management, diagnosis, treatment and ongoing wellness. Provides succinct and well referenced summaries of many aspects of SARS-CoV-2 infection in English and Spanish. It also provides links to other COVID-19 content hubs, including Springer Nature, Wiley, NEJM, BMJ, American Society for Microbiology, American College of Cardiology, etc.
Center for Science in the Public Interest
COVID-19 Evidence Hub. Center for Science in the Public Interest.
CSPI is a fifty-year old independent science-based consumer advocacy organization. This site provides a hub that aggregates more than 35 international databases related to evidence on diagnosis, vaccines, drug treatments, therapeutic interventions and public health measures for COVID-19.
Open Critical Care (OCC)
Open Critical Care Portal: The hub for critical care education tools
OCC is a collaborative recently initiated by the University of California San Francisco (UCSF), partnering with Brigham & Women’s Hospital (BWH) and other organizations such as Partners in Health. Their site is currently in beta mode and offers open access critical care educational resources styled for independent learning as well as institutional curricula. It includes a COVID-19 Guidelines Dashboard that aggregates recommendations from multiple health authorities into a consensus for each therapy, a series of detailed protocols developed at BWH on many aspects of COVID-19, and also apps applicable to acute respiratory failure in general.
Summaries of Special COVID-19 Topics
The Virus, The Vaccines, and The Variants
IARS COVID-19 Resource Newsletter, #98. Posted August 30, 2021. This brief review provides a concise explanation the relationship between the virus, the vaccines and the variants. 27 references.
[Author: Robert N. Sladen, MBChB, FCCM, on behalf of the IARS Scientific Advisory Board]
Vaccine-Induced Immune Thrombotic Thrombocytopenia (VITT)
IARS COVID-19 Resource Newsletter, #79. Posted April 21, 2021. This brief review provides a concise explanation of what is known about this rare but newsworthy vaccine induced problem. 5 references.
[Author: Robert N. Sladen MBChB, FCCM on behalf of the IARS Scientific Advisory Board]
Long COVID
IARS COVID-19 Resource Newsletter, #76. Posted April 12, 2021. This is an excellent summary of the prolonged impairment of functional capacity after COVID-19. 30 references.
[Author: Robert N. Sladen MBChB, FCCM on behalf of the IARS Scientific Advisory Board]
NIH Therapeutics Recommendations (Updated March 2, 2022)
Based upon data generated during the Omicron variant surge, the NIH has revised its recommendations for antiviral therapeutics for non-hospitalized patients with mild-moderate COVID-19 at high risk of progression to severe disease. The drugs are ranked by order of preference. For a full list, visit https://www.covid19treatmentguidelines.nih.gov/therapies/statement-on-therapies-for-high-risk-nonhospitalized-patients/.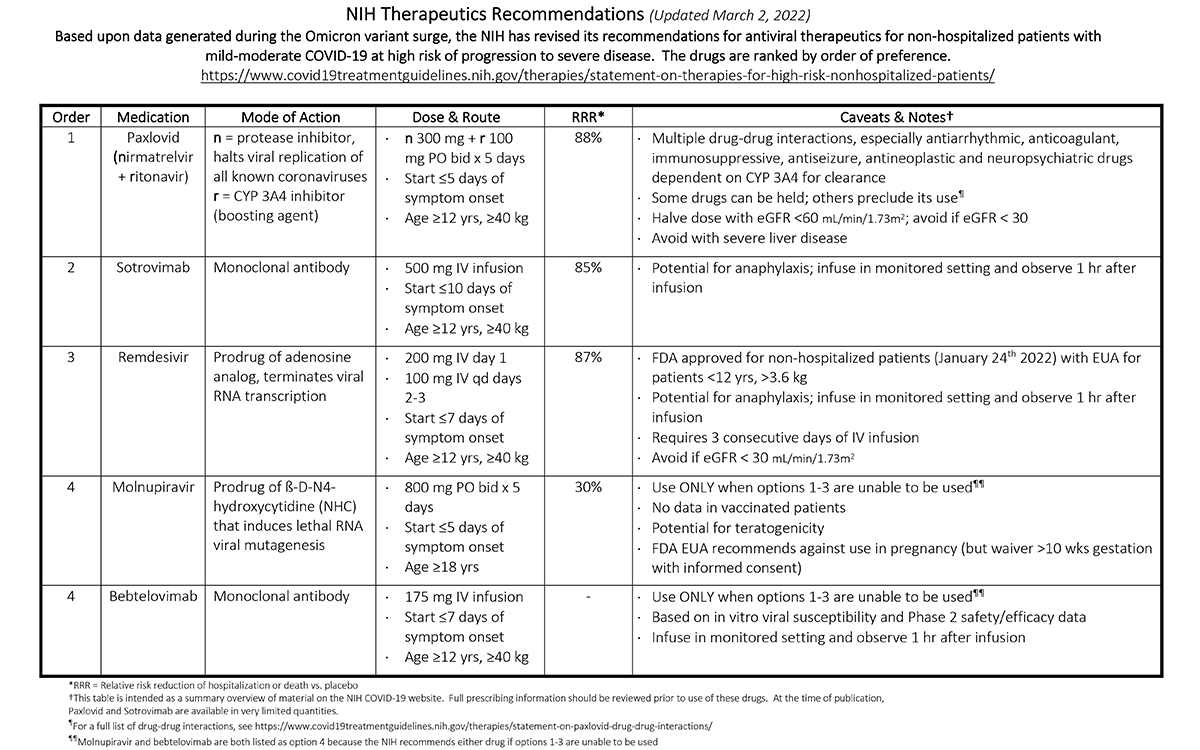
The material on this website is provided for informational purposes and does not constitute medical advice. New knowledge is added daily and may change over time. Opinions expressed should not be construed as representing IARS policy or recommendations. References and links to third parties do not constitute an endorsement or warranty by IARS.
International Anesthesia Research Society
